Rationale: An important element of the protection of patient safety, our highest priority, is preventing contamination and maintaining sterility, as applicable, for products or clinical materials. We have the important responsibility to assure controls are in place to prevent exposure to unintended and potentially harmful materials by patients being treated with products or participating in clinical trials. Equally important is the protection of personnel working with materials from exposure levels that could exceed safe limits.
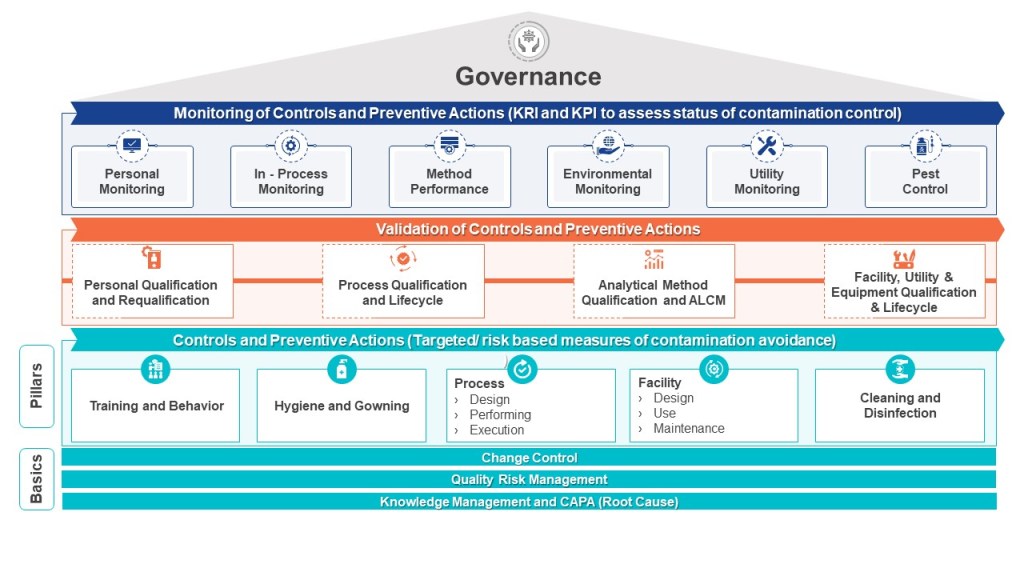
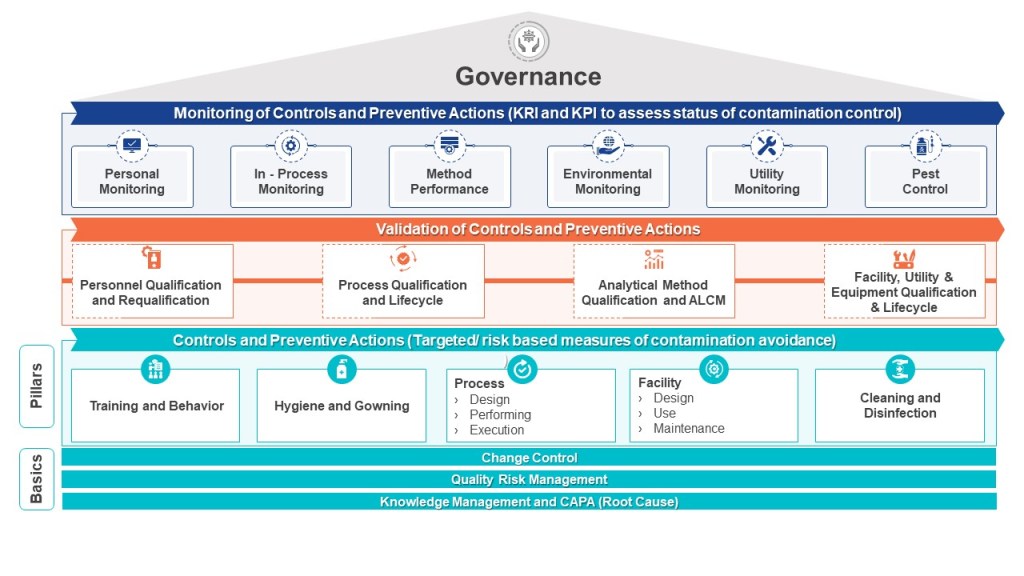
Policy objective: To define the expectations in implementing containment controls designed to minimize the likelihood of contamination and if applicable to assure the maintenance of sterility.
Procedures will be implemented to assure:
- Establishment of effective means to contain ingredients, in process and finished materials to the manufacturing equipment and containers designed for their use, and which prevent airborne or physical transmittal of foreign materials into ingredients, in process, or finished products or product contact surfaces.
- Application of risk based control mechanisms to establish steps to be taken to control cross contamination. Factors to be considered include, but may not be limited to, toxic risk of materials; physical properties that present contamination risk; product contact surfaces; use of lubricants; establishment and monitoring of air pressure differential cascades in manufacturing areas; filtration of air, water, steam, gases, and vacuum; the proper use of cleaning materials, sanitizers, and application of pesticides; and the use of personal protective equipment for employees who work with high risk materials.
- Classification of processing areas utilizing accepted international norms for viable and non-viable particulate levels
- Design of facilities and utilities to ensure appropriate contamination controls and if applicable aseptic conditions
- Definition of standards for and types of personal protective equipment (PPE) and procedures for donning PPE, plus additional personnel controls (such as hand washing and sanitization), and the exclusion of inappropriate materials (such as fiber shedding paper) as conditions of entry into classified areas.
- Establishment of alert and action levels of viable and nonviable particulate matter in air, water, gases, product contact surfaces and personnel, together with monitoring methods and frequency, and steps to be taken when such levels are exceeded.
- Assessment and validation of the effectiveness of containment controls, through methods such as periodic visualization of airflow patterns; water fills; media fills; and oversight of employee practices