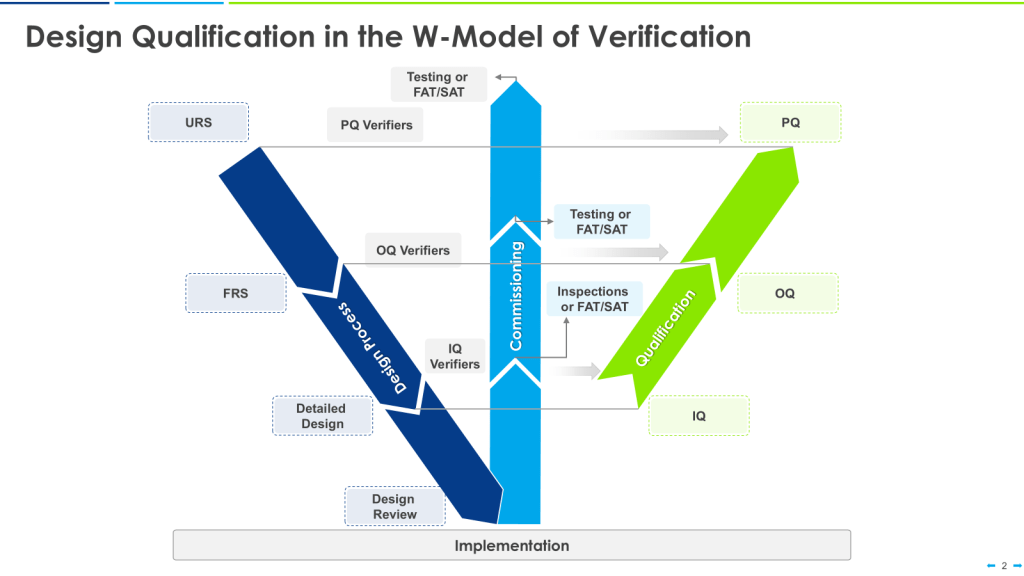
A critical step in ensuring the quality and safety of processes as part of verification is Design Review, which is sometimes expanded to Design Qualification.
Design Review: The Foundation of Quality
Design Review is a systematic, documented examination of a proposed design to evaluate its adequacy and identify potential issues early in the development process. Here’s how to conduct an effective Design Review:
- Plan Systematically: Schedule reviews at appropriate stages of development, ensuring they align with your project timeline.
- Involve the Right People: Include representatives from all relevant functions and an independent reviewer not directly responsible for the design stage being evaluated.
- Focus on Critical Aspects: Prioritize design elements that directly impact product quality and patient safety.
- Document Thoroughly: Record all findings, including the design under review, participants, date, and any proposed actions.
- Iterate as Needed: Conduct reviews iteratively as supplier design documents are published, allowing for early issue identification and correction.
Design Qualification: Verifying Suitability
Design Qualification (DQ) is the documented verification that the proposed design of facilities, equipment, or systems is suitable for its intended purpose. Here’s how to implement DQ effectively:
- Develop User Requirements: Create a detailed User Requirements Specification (URS) outlining what the equipment or system is expected to do.
- Create Functional Specifications: Translate user requirements into technical specifications that guide the design process.
- Perform Risk Assessment: Identify potential risks associated with the design and develop mitigation strategies.
- Review Design Specifications: Ensure the design meets all specified requirements, including GMP and regulatory standards.
- Document and Approve: Formally document the DQ process and obtain approval from key stakeholders, including quality assurance personnel.
Integrating Design Review and DQ
To maximize the effectiveness of these processes:
- Use a Risk-Based Approach: Prioritize efforts based on the level of risk to product quality and patient safety.
- Leverage Subject Matter Experts: Involve SMEs from the start to contribute their expertise throughout the process.
- Implement Change Management: Establish a robust system to manage design changes effectively and avoid late-stage issues.
- Ensure Quality Oversight: Have Quality Assurance provide oversight to maintain compliance with current regulations and GMP requirements.
- Document Comprehensively: Maintain thorough records of all reviews, qualifications, and decisions made during the process.
Implementing a systematic approach to Design Review and Design Qualification not only helps meet regulatory expectations but also contributes to operational efficiency and product excellence. As the pharmaceutical landscape evolves, staying committed to these foundational practices will remain crucial for success in this highly regulated industry.