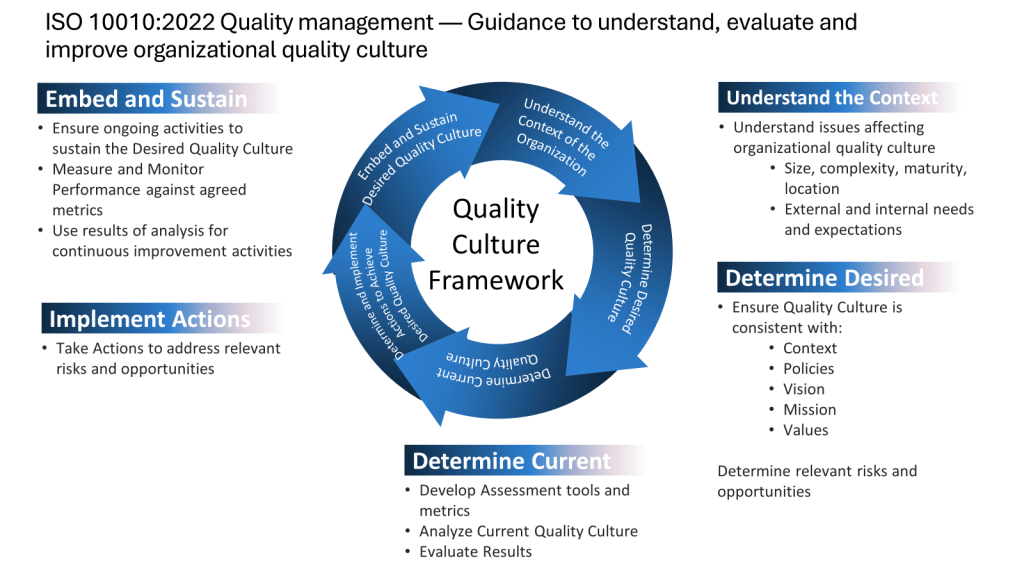
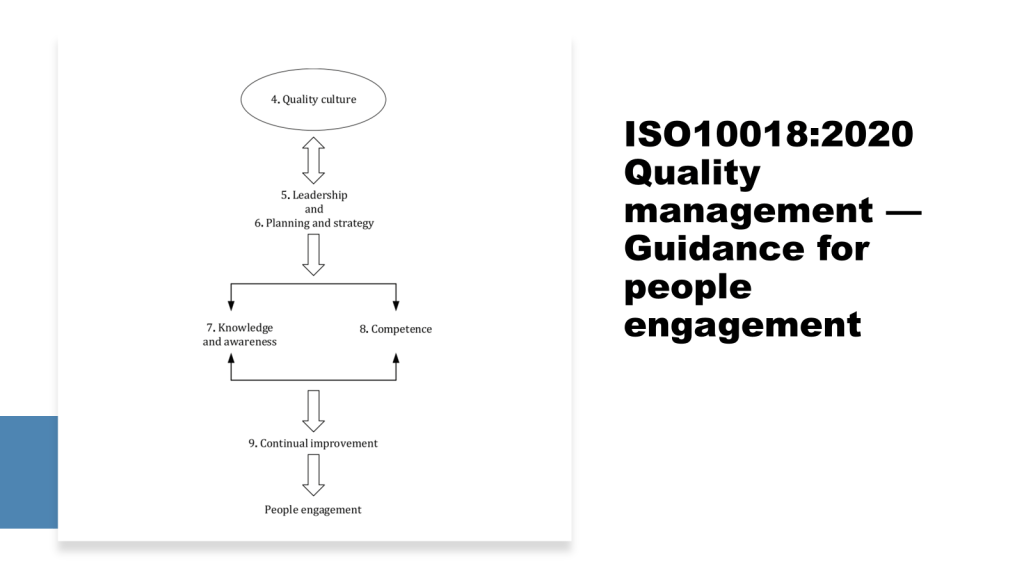
A consensus standards organization, also known as a voluntary consensus standards body, is an entity that develops and publishes technical standards through a collaborative, consensus-based process involving various stakeholders. Here are the key characteristics of consensus standards organizations:
- Voluntary participation: Involvement in the standards development process is voluntary for interested parties.
- Consensus-based approach: Standards are developed through a process that seeks general agreement among participants, considering the views of all parties and reconciling conflicting arguments.
- Openness: The procedures and processes for developing standards are open to interested parties, providing meaningful opportunities for participation on a non-discriminatory basis.
- Balance: The standards development process aims to achieve balance among different stakeholder groups, ensuring no single interest dominates.
- Due process: The organization follows established procedures that include provisions for appeals and addressing objections.
- Transparency: The procedures for developing standards and the standards themselves are transparent and accessible.
- Non-profit status: Many consensus standards organizations operate as non-profit entities.
- Diverse stakeholder involvement: Participants typically include industry experts, government representatives, academics, and consumer groups.
- Accreditation: In some cases, these organizations may be accredited by national bodies (e.g., ANSI in the United States) to ensure they follow proper procedures.
- Wide range of applications: Consensus standards can cover various fields, including product specifications, testing methods, management systems, and more.
Examples of well-known consensus standards organizations include:
- International Organization for Standardization (ISO)
- American National Standards Institute (ANSI)
- ASTM International (formerly American Society for Testing and Materials)
- British Standards Institution (BSI)
These organizations play a crucial role in promoting quality, safety, and interoperability across various industries and sectors by developing widely accepted standards through collaborative processes.
The Unique Role of Inter-Governmental Agencies in Pharmaceutical Standards
While discussing consensus standard organizations, it’s important to highlight a distinct category that operates similarly but doesn’t quite fit the traditional mold: inter-governmental agencies like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the Pharmaceutical Inspection Co-operation Scheme (PIC/S).
These organizations share some key characteristics with consensus standard bodies:
- They focus on harmonization efforts in the pharmaceutical industry.
- They operate internationally, involving multiple countries and regulatory authorities.
- They provide frameworks for collaboration among stakeholders.
However, ICH and PIC/S differ from typical consensus standard organizations in several ways:
- Membership: They primarily comprise regulatory authorities rather than a broad range of industry stakeholders.
- Authority: While not legally binding, their guidelines and standards often carry significant weight with regulatory bodies worldwide.
These organizations play a crucial role in shaping global pharmaceutical regulations, bridging the gap between formal regulatory requirements and industry-led standards. Their work complements that of traditional consensus standard organizations, contributing to a more cohesive and harmonized global regulatory environment for pharmaceuticals.