The Validation Master Plan (VMP) and Validation Plan (VP) are integral to the validation process but differ significantly in their scope, detail, and application. The VMP provides a strategic and comprehensive outline for validation activities (often capturing the whole commissioning/qualification/validation lifecycle) across an organization, ensuring compliance and coherence. The VP, derived from the VMP, focuses on specific validation projects, detailing the procedures, responsibilities, and requirements needed to achieve compliance for those specific systems or projects.
Validation Master Plan (VMP)
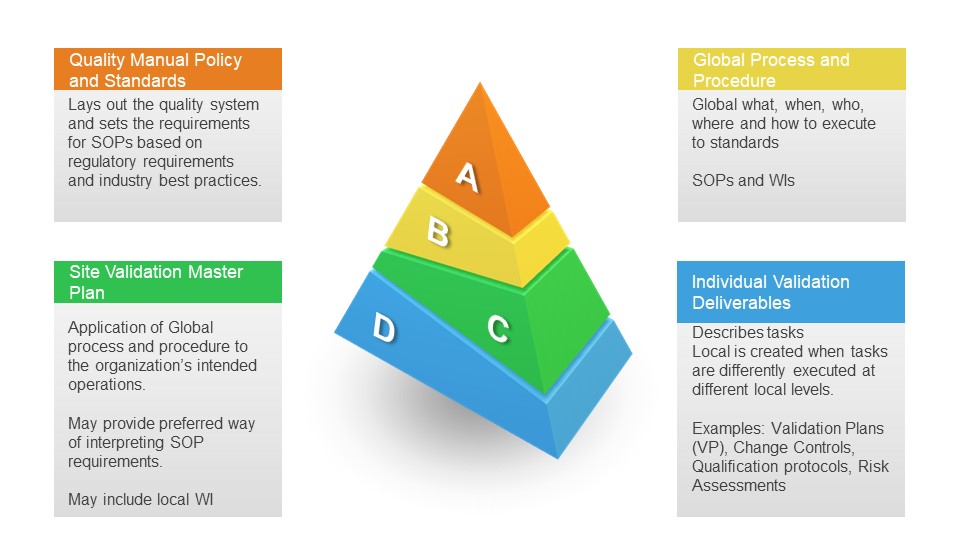
A Validation Master Plan is a high-level document that outlines the overall validation strategy for an entire site or organization. It is comprehensive and covers all aspects of validation activities across various departments and systems within the organization. The VMP is designed to ensure that all components of the validation process are appropriately planned, executed, and maintained to meet regulatory compliance requirements.
Key characteristics of a VMP include:
- Scope and Purpose: It defines the scope and objectives of all validation activities within the organization.
- Strategy and Approach: It outlines the validation strategy and approach, including integrating Good Manufacturing Practices (GMP).
- Responsibilities: It details the organizational structure and responsibilities for validation activities.
- Documentation: It references all applicable protocols, reports, and related documents.
- Compliance and Review: It includes compliance requirements and specifies the frequency of reviews and updates to ensure the plan remains current.
A Subvalidation Master Plan (sVMP) is a deep dive into a specific area or validation, such as the analytical method lifecycle.
The purpose of a Validation Master Plan (VMP) is multifaceted, primarily serving as a comprehensive document that outlines the strategy for validation activities within an organization. It is designed to ensure that all validation processes are conducted correctly and comply with regulatory standards.
Here are the key purposes of a VMP:
- Documentation of Compliance Requirements: The VMP documents the organization’s compliance requirements, ensuring that all validation activities meet the necessary regulatory standards.
- Strategic Planning: Acts as a roadmap for validation, detailing what, how, and when validation activities will be executed. This includes the lifecycle of the manufacturing validation process and integrates Good Manufacturing Practices (GMP).
- Resource Planning: The VMP identifies anticipated resource needs and provides key input into scheduling project timelines, which is crucial for efficient validation execution.
- Control and Direction: The VMP controls and defines different parts of the production process to ensure consistency over time and directs validation strategies for instruments and systems.
- Risk Mitigation: The VMP helps mitigate risks associated with product manufacturing by outlining the validation approach and specific validation activities.
- Educational Tool: The VMP informs and educates senior management and other stakeholders about the importance of validation in terms of its impact on product quality, thereby fostering an understanding and support for validation activities.
- Regulatory Audit Support: It provides essential documentation regulators require during an audit, demonstrating the organization’s control over quality and compliance with GMPs.
- Organizational Alignment: The VMP enables stakeholders within the organization to unify around the details of the validation strategy, eliminating ambiguity and justifying validation activities internally and externally.
The Validation Master Plan is crucial for ensuring that all aspects of validation are planned, executed, and documented in accordance with regulatory requirements and organizational goals. It serves as a compliance tool and a strategic guide for managing and conducting validation activities effectively.
Validation Plan (VP)
A Validation Plan (VP) is more specific and detailed than a VMP and is typically written for a particular validation project or system. The VP focuses on the specific validation activities for individual pieces of equipment, systems, or processes and is derived from the broader directives set out in the VMP.
Key characteristics of a VP include:
- Detailed Scope and Objectives: It describes what is to be validated, the specific tasks to be performed, and the expected outcomes.
- Project-Specific Details: These include timelines, specific procedures, and responsibilities for the particular validation project.
- Risk Assessments and Requirements: It details the risk assessments, quality parameters, and regulatory requirements specific to the system or project being validated.
Differences and Relationship
Level of Detail: The VMP is a high-level document that provides an overarching framework and strategy for validation activities across an organization. In contrast, a VP is a detailed, project-specific document that outlines the execution of validation activities for specific systems or projects.
Purpose and Use: The VMP sets the stage for all validation efforts within an organization and ensures consistency and compliance with industry standards. The VP, derived from the VMP, focuses on specific validation tasks and how they will be accomplished.
Scope: While the VMP covers an organization’s entire validation program, a VP is limited to a particular project or system.
Periodic Review and Updates
A Validation Master Plan (VMP) should be reviewed and updated regularly to remain current and effective. The specific frequency of these reviews can vary depending on the organization’s needs, the complexity of the systems, and regulatory requirements. However, it is generally recommended that a VMP be reviewed at least annually.
This annual review is crucial to address any changes in the manufacturing process, regulatory updates, or modifications in the validation strategy. The review process should include evaluating the progress of validation activities, assessing the impact of any changes in the process or equipment, and updating the plan to reflect new or altered validation requirements.
Additionally, the VMP should be updated whenever significant changes occur that could affect the validation status of the systems or processes described in the plan. This could include major equipment upgrades, product design changes, or regulatory standard shifts.
Validation Plans (VP) should be revised based on changes in the project’s scope. Sometimes, a VP may be opened for an extended period of time for a complex project, in which case it should be evaluated for accuracy and completeness based on the project lifecycle.

2 thoughts on “Validation Planning in the Quality System”