ICH Q10 “Pharmaceutical Quality System” describes a lifecycle approach, from development through product discontinuation. The knowledge about a pharmaceutical product and the processes required to reliably produce that product starts with product and process development. An effective pharmaceutical quality system (PQS) uses the knowledge acquired throughout the lifecycle of the product, builds on that knowledge, and applies it to:
- Other stages of the product lifecycle
- Other product lifecycles
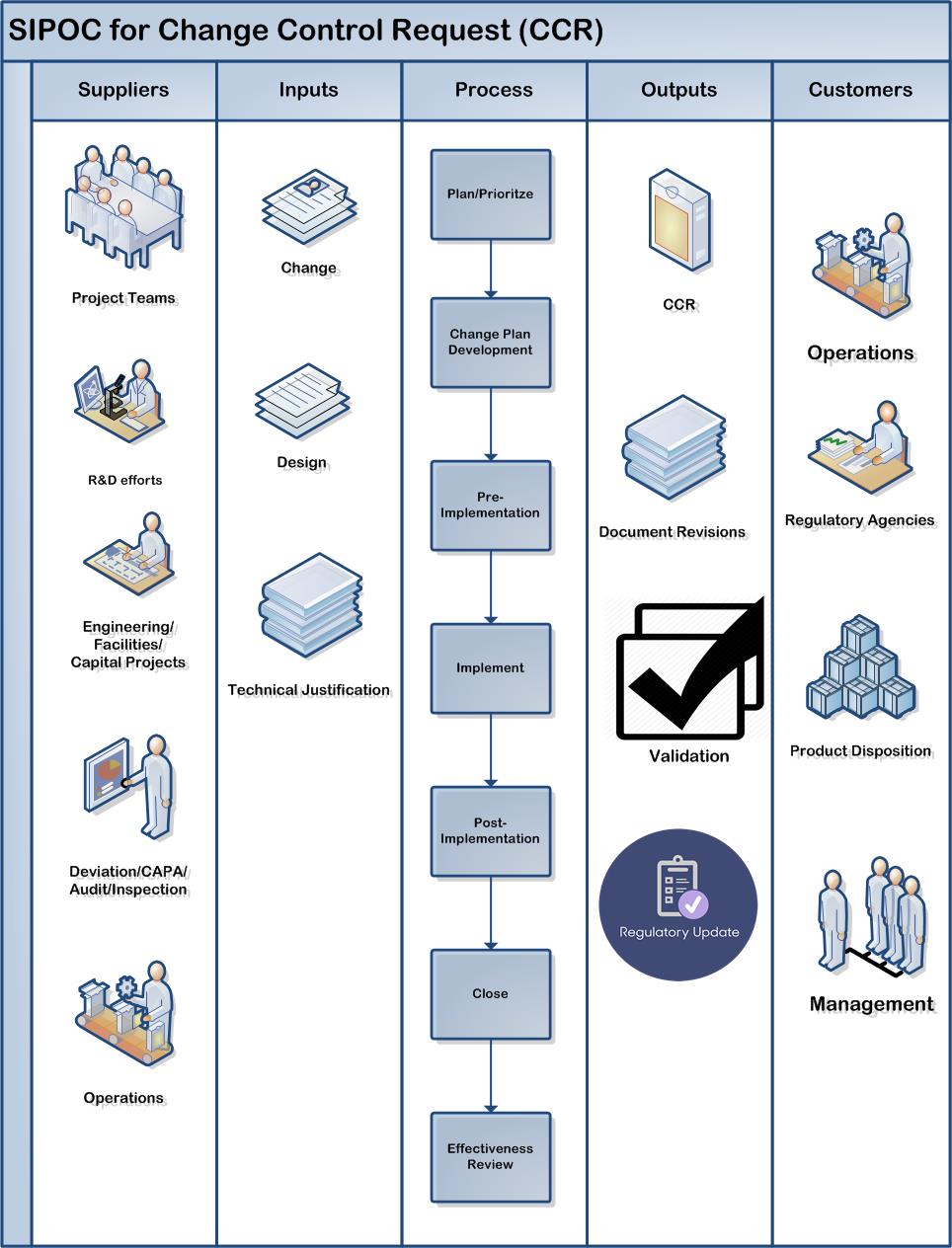
A change management system is defined as an important element of a PQS as seen in this figure reproduced from ICH Q10.
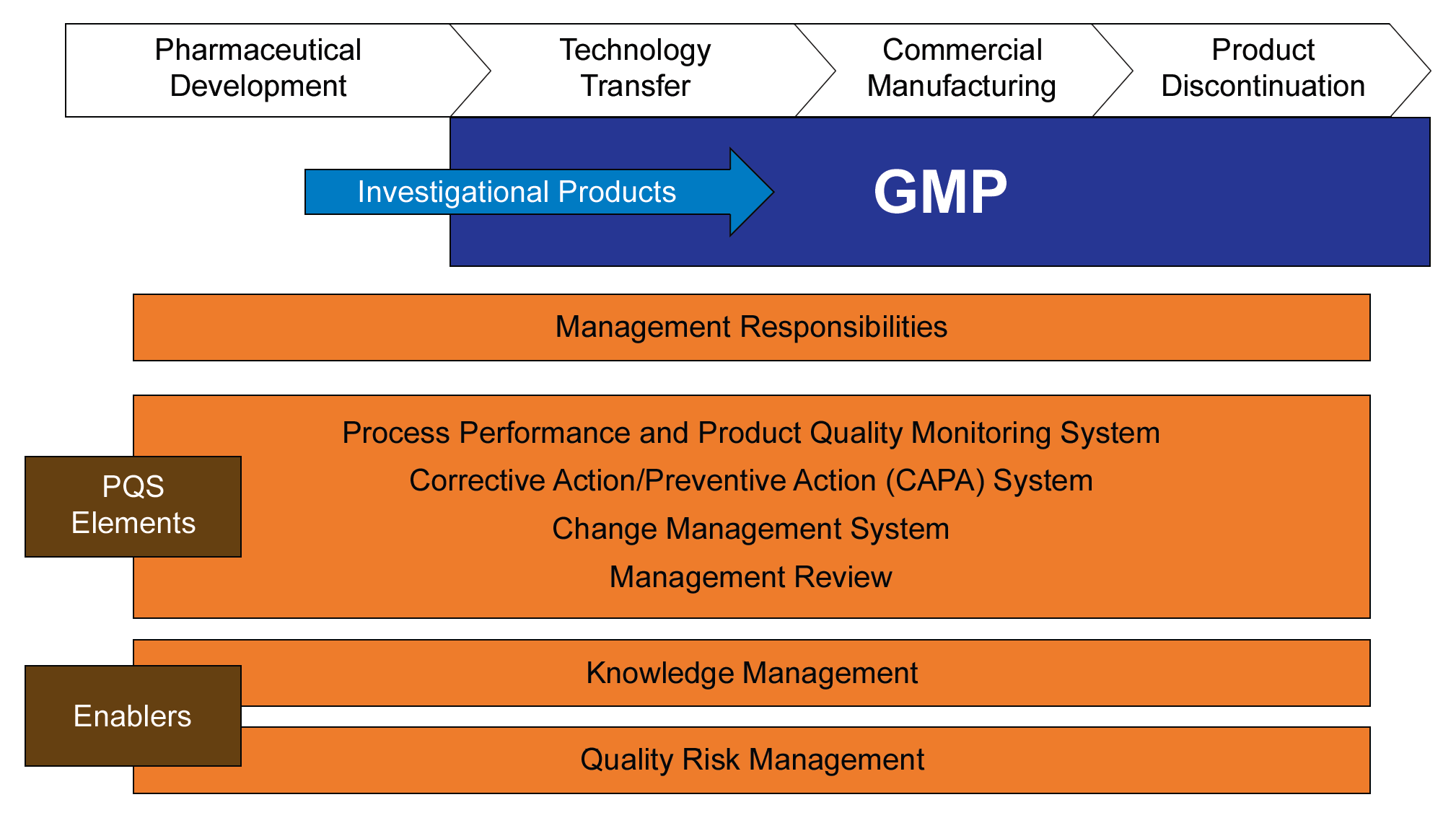
There are two enablers to this quality system model, knowledge management and risk management. The thing about those enablers is that they are really intertwined. Or put another way, risk management is a powerful way to make use of your knowledge.
ICHQ12 “Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management” (in draft) expands on knowledge management and provides more examples of its use. The below illustration is an adaptation of one found in the draft Q12.

There are many ways to tap into knowledge management in change management. The subject matter experts are critical, as is checklists and risk ranking and filtering tools. Knowledge should drive the development of an effectiveness review.
One of my favorite is the Living Risk Assessment approach. Living risk assessments are a holistic view of a system, product, or process in an effort to prevent risk realization. They are updated throughout the product /system lifecycle to continuously assess risks that may arise or change.
In the context of change management, the living risk assessment is both an input and an output. A rigorous, maintained, living risk assessment allows us to prospectively mitigate potential risks as part of our change management program.
Living Risk Assessments have a schedule, a review period (for example, once a year) to evaluate how risk has changed, drawing from all the sources of knowledge. It is also important to have a way to trigger adhoc reviews (for example, major process changes or critical deviations).

In my ASQ World Conference workshop I will be going into more detail on knowledge management, risk management and the pharmaceutical quality system. I’ll also be discussing what non-Pharma companies can learn from the PQS.