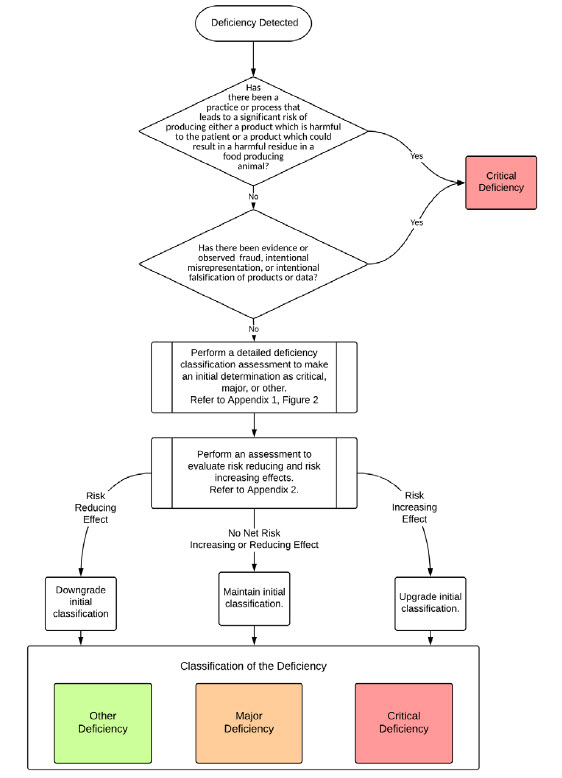
The Pharmaceutical Inspection Convention Cooperation Scheme (PIC/S) on 01-Jan-2019 released a long-awaited guidance to help regulators harmonize the classification and reporting of good manufacturing practice (GMP) deficiency outcomes from inspections. The guidance is designed as a “tool to support the risk-based classification of GMP deficiencies from inspections and to establish consistency amongst inspectorates.”
| PI 040-1 “Guidance on Classification of GMP Deficiencies“ |