Normally, when I write a blog post, I include the graphics, but I decided to separate them out to show some of my thought processes for designing slides.
I start with a nice slide that introduces the topic I am going to discuss, introducing the main concept, the Validation Master Plan (VMP), and the Validation Plan (VP.)

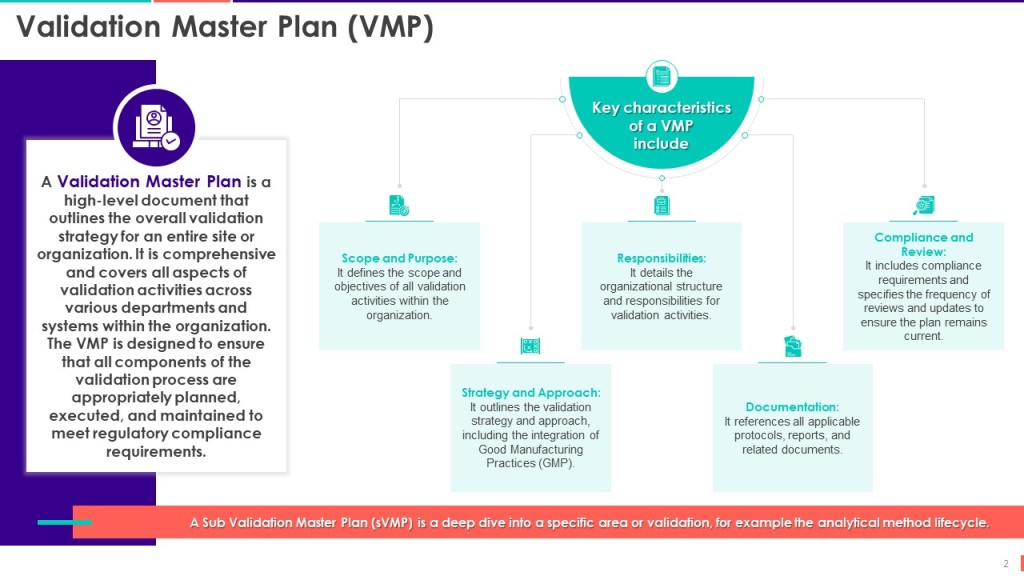
My next slide details the Validation Master Plan in more depth, covering the VMP’s core characteristics.
Then dive into the reasons for having a VMP.

Then cover the Validation plan characteristics.


These are still rather wordy, and I think the last slide can be divided into two. But I have a pretty good training here.

Hello Jeremiah,
Interesting articles…they always are!
Coincidence, I’m currently having a problem involving validation. I have been in quality field for several years but I am not in the pharmaceutical or medical sector and I must admit the concept is new to me.
By the way, the slides are interesting but the text is often too small and the resolution is poor. Wouldn’t it be possible to have a link to download these?
Regards
Francois
LikeLike
Sure, here’s a link to the ppt for these: https://investigationsquality.com/wp-content/uploads/2024/05/vmp-and-vp-graphics.pptx
LikeLike
Let me know if you have any specific validation questions! Always looking for topics.
LikeLike
Hello Jeremiah,
Thanks for the link. Is there a “special place” where we could download as needed (versus having to ask) ?
A bit about me. I’m on LinkedIn and also member of ASQ. As you will be able to see, I work for Gentec (www.gentec.ca). We’re a small shop, an EMS (Electronic Manufacturing Services). There is some interesting pictures on the web site.
We provide a small board (a flex with a chip on it to be more precise) to a customer nearby. He puts this flex in a tube and this tube is put in bodies to measure blood pressure.
One of their customers buys these products directly from them, but in order to be able to demonstrate that they have a 2nd source, they also buy from us (even if it’s basically the same source)!
In short, this client – that I won’t name! 😉 – was purchased by J&J and I recently received a sort of “quality contract” of which 3/4 do not apply (requirement type from pharma or medical) – including validation. I don’t know yet how I’m going to respond to them but for “validation”, I can’t say that I’ve seen that or heard about that except from a medical sector … and I’m not in this (rather in the electronics field). To give you an idea, I assemble boards that are used in industrial computers.
I’m not saying that we couldn’t use validation, but you will understand that its not in our core practice …
If you have suggestions, advice, etc., I’ll take them ! 😉
Thank you in advance.
Congratulations again on your blog which I read with keen interest! 🙂
Francois Denis
Quebec City, QC, Canada
(fdenis@gentec.ca / fdenisqc@gmail.com)
LikeLike
That link goes to the file directly. I probably should create some sort of central file repository sometime. Thanks for the suggestion!
Okay, so you are a component manufacturer who has a client that does a medical device. Fun area, especially as the big companies (like J&J) like to push their responsibilities lower in the supply chain.
I do have some thoughts, let me see about putting them in a coherent way and getting something out next week.
LikeLike
Okay here are my top level thoughts on what the regulations and ISO 13485 require: https://investigationsquality.com/2024/05/25/component-manufacturers-validation-requirements/
LikeLike
Ok I saw it. Thanks. Interesting.
You had an interesting insight in your short answer. “(…) what the regulations and ISO 13485 require”. I’m not ISO 13485 (only ISO 9001).
Could I conclude from this that validation does not apply to me? Couldn’t it be done by the customer? In fact, the customer probably made a “mistake” in coming to us. He should have ensured that his supplier is ISO 13485!
Thanks again for the info. Still very interesting.
Jeremiah, you should have a YouTube channel: it would make the transfer of information even more interesting! 😉
François
LikeLike
I recommend doing qualification and then valdiating the process in a risk based approach as it sounds like you are delivering a component that impacts the safety, efficacy, or critical performance characteristics of the finished medical device.
LikeLike