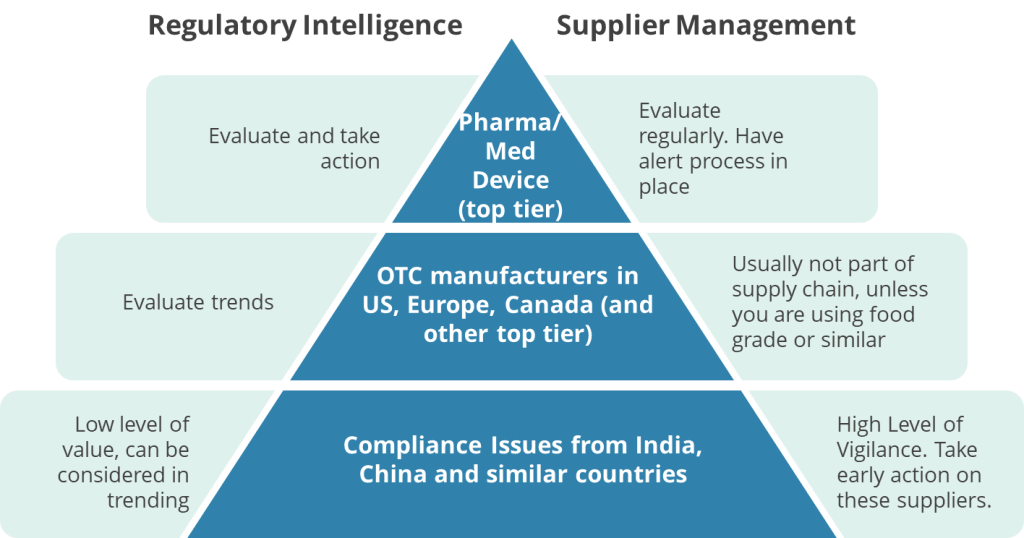
I have written in the past that I do not treat all regulatory compliance actions with equal importance. Not every Form 483 or Warning Letter carries the same weight; their significance is determined by the nature of the company involved.
Take the April 2025 Warning Letter to Cosco International, for example. One might quickly react with, “Holy cow! No process validation or cleaning validation—how is this even possible?” This could spark an exhaustive discussion about why these regulations have been in place for 30 years and the urgent need for companies to comply. But frankly, nothing really valuable to a company that already realizes they need to do process validation.
Yet this Warning Letter highlights a fundamental misunderstanding among companies regarding the difference between a cosmetic and a drug. As someone who reads Warning Letters, this seems to be a fairly common problem.
Key Regulatory Distinctions
- Cosmetics: Products intended solely for cleansing, beautifying, or altering the appearance without affecting bodily functions are regulated as cosmetics under the FDA. These are not required to undergo premarket approval, except for color additives.
- Drugs: Products intended to diagnose, cure, mitigate, treat, or prevent disease or that affect the structure or function of the body (such as blocking sweat glands) are regulated as drugs. This includes antiperspirants, regardless of their application site.
So not really all that interesting from a biotech perspective, but a fascinating insight to some bad trends if I was on the consumer goods side of the profession.
But, as I discussed, there is value from reading these holistically, for what they tell us regulators are thinking. In this case, there is a nice little set of bullet points on what is bare minimum in cleaning validation.