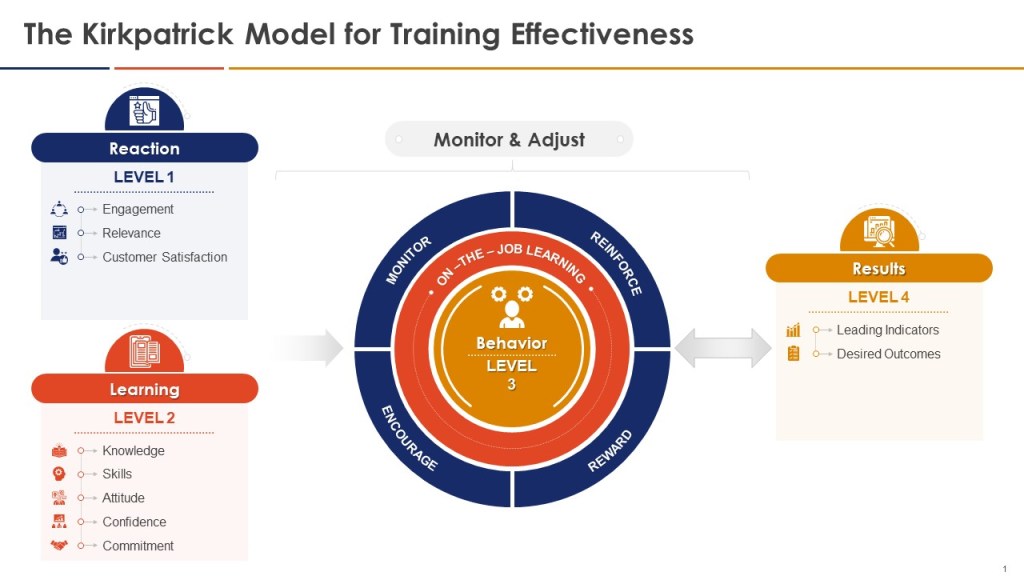
When thinking about the training program you can add the Kilpatrick model to the mix and build from there. This allows a view across the training system to drive for an effective training program.
GMP Training Metrics Framework Aligned with Kirkpatrick’s Model
| Kirkpatrick Level | Category | Metric Type | Example | Purpose | Data Source | Regulatory Alignment |
|---|---|---|---|---|---|---|
| Level 1: Reaction | KPI | Leading | % Training Satisfaction Surveys Completed | Measures engagement and perceived relevance of GMP training | LMS (Learning Management System) | ICH Q10 Section 2.7 (Training Effectiveness) |
| KRI | Leading | % Surveys with Negative Feedback (<70%) | Identifies risk of disengagement or poor training design | Survey Tools | FDA Quality Metrics Reporting (2025 Draft) | |
| KBI | Leading | Participation in Post-Training Feedback | Encourages proactive communication about training gaps | Attendance Logs | EU GMP Chapter 2 (Personnel Training) | |
| Level 2: Learning | KPI | Leading | Pre/Post-Training Quiz Pass Rate (≥90%) | Validates knowledge retention of GMP principles | Assessment Software | 21 CFR 211.25 (Training Requirements) |
| KRI | Leading | % Trainees Requiring Remediation (>15%) | Predicts future compliance risks due to knowledge gaps | LMS Remediation Reports | FDA Warning Letters (Training Deficiencies) | |
| KBI | Lagging | Reduction in Knowledge Assessment Retakes | Validates long-term retention of GMP concepts | Training Records | ICH Q7 Section 2.12 (Training Documentation) | |
| Level 3: Behavior | KPI | Leading | Observed GMP Compliance Rate During Audits | Measures real-time application of training in daily workflows | Audit Checklists | FDA 21 CFR 211 (cGMP Compliance) |
| KRI | Leading | Near-Miss Reports Linked to Training Gaps | Identifies emerging behavioral risks before incidents occur | QMS (Quality Management System) | ISO 9001:2015 Clause 10.2 (Nonconformity) | |
| KBI | Leading | Frequency of Peer-to-Peer Knowledge Sharing | Encourages a culture of continuous learning and collaboration | Meeting Logs | ICH Q10 Section 3.2.3 (Knowledge Management) | |
| Level 4: Results | KPI | Lagging | % Reduction in Repeat Deviations Post-Training | Quantifies training’s impact on operational quality | Deviation Management Systems | FDA Quality Metrics (Batch Rejection Rate) |
| KRI | Lagging | Audit Findings Related to Training Effectiveness | Reflects systemic training failures impacting compliance | Regulatory Audit Reports | EU GMP Annex 15 (Qualification & Validation) | |
| KBI | Lagging | Employee Turnover | Assesses cultural impact of training on staff retention | HR Records | ICH Q10 Section 1.5 (Management Responsibility) |
Kirkpatrick Model Integration
- Level 1 (Reaction):
- Leading KPI: Track survey completion to ensure trainees perceive value in GMP content.
- Leading KRI: Flag facilities with >30% negative feedback for immediate remediation .
- Level 2 (Learning):
- Leading KPI: Require ≥90% quiz pass rates for high-risk roles (e.g., aseptic operators) .
- Lagging KBI: Retake rates >20% trigger refresher courses under EU GMP Chapter 3 .
- Level 3 (Behavior):
- Leading KPI: <95% compliance during audits mandates retraining per 21 CFR 211.25 .
- Leading KRI: >5 near-misses/month linked to training gaps violates FDA’s “state of control” .
- Level 4 (Results):
- Lagging KPI: <10% reduction in deviations triggers CAPA under ICH Q10 Section 4.3 .
- Lagging KRI: Audit findings >3/year require FDA-mandated QMS reviews .
Regulatory & Strategic Alignment
- FDA Quality Metrics: Level 4 KPIs (e.g., deviation reduction) align with FDA’s 2025 focus on “sustainable compliance” .
- ICH Q10: Level 3 KBIs (peer knowledge sharing) support “continual improvement of process performance” .
- EU GMP: Level 2 KRIs (remediation rates) enforce Annex 11’s electronic training documentation requirements .
By integrating Kirkpatrick’s levels with GMP training metrics, organizations bridge knowledge acquisition to measurable quality outcomes while meeting global regulatory expectations.

Think the reference should be Q7 Section 3.12. Thanks for these posts. Have just found this blog but am a daily reader now!
LikeLike