The quality management landscape has always been a battlefield of competing priorities, but today’s environment demands more than just compliance-it requires systems that thrive in chaos. For years, frameworks like VUCA (Volatility, Uncertainty, Complexity, Ambiguity) have dominated discussions about organizational resilience. But as the world fractures into what Jamais Cascio terms a BANI reality (Brittle, Anxious, Non-linear, Incomprehensible), our quality systems must evolve beyond 20th-century industrial thinking. Drawing from my decade of dissecting quality systems on Investigations of a Dog, let’s explore how these frameworks can inform modern quality management systems (QMS) and drive maturity.
VUCA: A Checklist, Not a Crutch
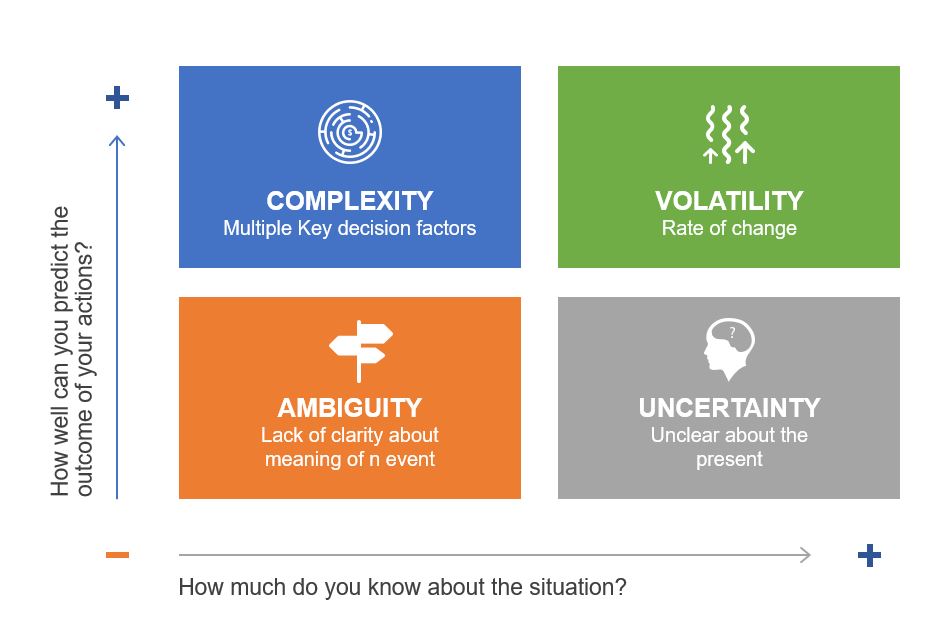
VUCA entered the lexicon as a military term, but its adoption by businesses has been fraught with misuse. As I’ve argued before, treating VUCA as a single concept is a recipe for poor decisions. Each component demands distinct strategies:
Volatility ≠ Complexity
Volatility-rapid, unpredictable shifts-calls for adaptive processes. Think of commodity markets where prices swing wildly. In pharma, this mirrors supply chain disruptions. The solution isn’t tighter controls but modular systems that allow quick pivots without compromising quality. My post on operational stability highlights how mature systems balance flexibility with consistency.
Ambiguity ≠ Uncertainty
Ambiguity-the “gray zones” where cause-effect relationships blur-is where traditional QMS often stumble. As I noted in Dealing with Emotional Ambivalence, ambiguity aversion leads to over-standardization. Instead, build experimentation loops into your QMS. For example, use small-scale trials to test contamination controls before full implementation.
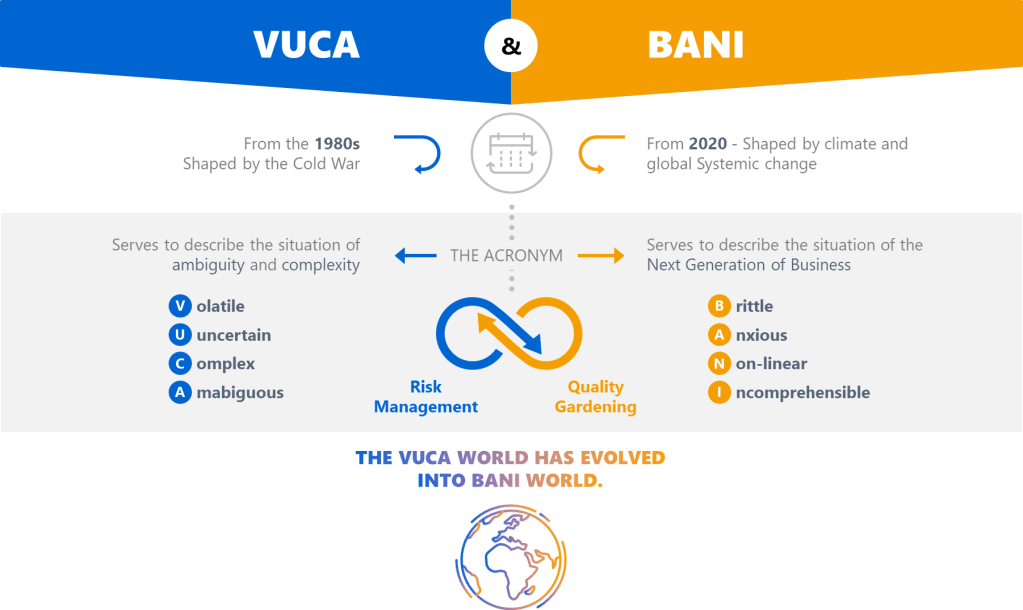
BANI: The New Reality Check
Cascio’s BANI framework isn’t just an update to VUCA-it’s a wake-up call. Let’s break it down through a QMS lens:
Brittle Systems Break Without Warning
The FDA’s Quality Management Maturity (QMM) program emphasizes that mature systems withstand shocks. But brittleness lurks in overly optimized processes. Consider a validation program that relies on a single supplier: efficient, yes, but one disruption collapses the entire workflow. My maturity model analysis shows that redundancy and diversification are non-negotiable in brittle environments.
Anxiety Demands Psychological Safety
Anxiety isn’t just an individual burden, it’s systemic. In regulated industries, fear of audits often drives document hoarding rather than genuine improvement. The key lies in cultural excellence, where psychological safety allows teams to report near-misses without blame.
Non-Linear Cause-Effect Upends Root Cause Analysis
Traditional CAPA assumes linearity: find the root cause, apply a fix. But in a non-linear world, minor deviations cascade unpredictably. We need to think more holistically about problem solving.
Incomprehensibility Requires Humility
When even experts can’t grasp full system interactions, transparency becomes strategic. Adopt open-book quality metrics to share real-time data across departments. Cross-functional reviews expose blind spots.
Building a BANI-Ready QMS
From Documents to Living Systems
Traditional QMS drown in documents that “gather dust” (Documents and the Heart of the Quality System). Instead, model your QMS as a self-adapting organism:
- Use digital twins to simulate disruptions
- Embed risk-based decision trees in SOPs
- Replace annual reviews with continuous maturity assessments
Maturity Models as Navigation Tools
A maturity model framework maps five stages from reactive to anticipatory. Utilizing a Maturity model for quality planning help prepare for what might happen.
Operational Stability as the Keystone
The House of Quality model positions operational stability as the bridge between culture and excellence. In BANI’s brittle world, stability isn’t rigidity-it’s dynamic equilibrium. For example, a plant might maintain ±1% humidity control not by tightening specs but by diversifying HVAC suppliers and using real-time IoT alerts.
The Path Forward
VUCA taught us to expect chaos; BANI forces us to surrender the illusion of control. For quality leaders, this means:
- Resist checklist thinking: VUCA’s four elements aren’t boxes to tick but lenses to sharpen focus.
- Embrace productive anxiety: As I wrote in Ambiguity, discomfort drives innovation when channeled into structured experimentation.
- Invest in sensemaking: Tools like Quality Function Deployment help teams contextualize fragmented data.
The future belongs to quality systems that don’t just survive chaos but harness it. As Cascio reminds us, the goal isn’t to predict the storm but to learn to dance in the rain.
For deeper dives into these concepts, explore my series on VUCA and Quality Systems.