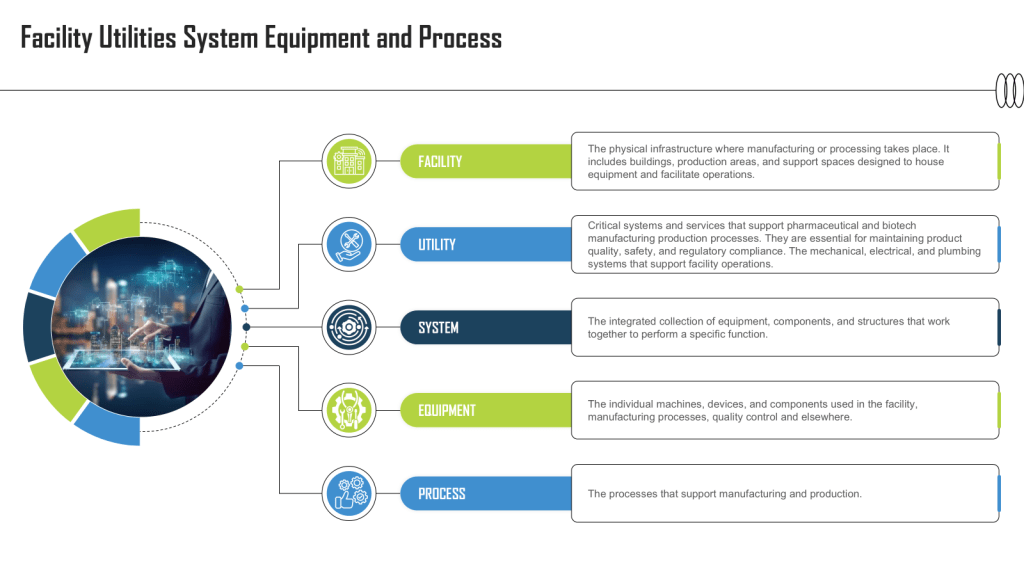
When considering the engagement of an external consultant for your facility, the decision should not be taken lightly. Consultants can provide invaluable insights when addressing compliance gaps, resolving environmental control issues, or conducting design reviews. However, the real value lies in their ability to bring expertise and actionable solutions tailored to your specific needs. To ensure this, assessing their relevant expertise and experience is paramount.
The first step in evaluating a consultant’s expertise is to scrutinize their professional background and track record. This involves examining their history of projects within your industry and determining whether they have successfully addressed challenges similar to yours. For instance, if you are dealing with deviations in environmental monitoring trends, you should confirm that the consultant has prior experience diagnosing and resolving such issues in facilities governed by comparable regulatory frameworks. Look for evidence of their familiarity with regulations and standards such as FDA 21 CFR Part 211 or ISO 14644 for cleanroom environments. Additionally, assess whether they have worked with facilities of a similar scale and complexity to yours—what works for a small-scale operation may not translate effectively to a larger, more intricate system.
To gain deeper insights into their qualifications, ask targeted questions during the evaluation process. For example:
- “Can you describe a recent project where you addressed similar challenges? What were the outcomes?”
- “How do you approach identifying root causes in complex systems?”
- “What methodologies or tools do you use to ensure compliance with regulatory standards?”
These questions not only help verify their technical knowledge but also reveal their problem-solving approach and adaptability.
Another critical aspect of assessing expertise is understanding their familiarity with current regulations and industry trends. A consultant who actively engages with updated guidelines from regulatory bodies like the FDA or EMA demonstrates a commitment to staying relevant. You might ask: “How do you stay informed about changes in regulations or advancements in technology that could impact our operations?” Their response can indicate whether they are proactive in maintaining their expertise or rely on outdated practices.
Experience is equally important in assessing whether a consultant can deliver practical, actionable recommendations. Review case studies or examples of past work that demonstrate measurable results—such as improved compliance rates, reduced deviations, or enhanced operational efficiency. Requesting references from previous clients is another effective way to validate their claims. When speaking with references, inquire about the consultant’s ability to communicate effectively, collaborate with internal teams, and deliver results within agreed timelines.
Ultimately, assessing expertise and experience requires a thorough evaluation of both technical qualifications and practical application. By asking detailed questions and reviewing tangible evidence of success, you can ensure that the consultant you hire has the skills and knowledge necessary to address your facility’s unique challenges effectively.
Companies that have participated in GMP remediation in response to warning letters or consent decrees offer a unique perspective on the intricacies of the facility. This experience allows them to:
- Identify systemic issues more effectively: Remediation veterans are better equipped to recognize underlying problems that may not be immediately apparent, having seen how seemingly minor issues can cascade into major compliance failures.
- Understand regulatory expectations: Direct experience with regulatory agencies during remediation provides insight into their thought processes, priorities, and interpretation of GMP requirements.
- Implement sustainable solutions: Those who have been through remediation understand the importance of addressing root causes rather than applying superficial fixes, ensuring long-term compliance.
- Prioritize effectively: Experience helps in distinguishing between critical issues that require immediate attention and those that can be addressed over time, allowing for more efficient resource allocation
Questions to Ask During Evaluation
To identify the best fit for your needs, ask potential consultants these critical questions:
- Can you provide examples of similar projects you’ve completed?
- This helps verify their experience with challenges of GMP facilities.
- Look for previous remediation experience
- What methodologies do you use?
- Ensure their approach aligns with your facility’s operational style and regulatory requirements.
- How do you ensure actionable recommendations?
- Look for consultants who provide clear implementation plans rather than vague advice.
- How do you handle confidentiality?
- Confirm safeguards are in place to protect sensitive information.
- Can you share references from past clients?
- Contact references to assess reliability, responsiveness, and outcomes achieved.
- What is your communication style?
- Evaluate their ability to provide timely updates and collaborate effectively with your team.
Ensuring Actionable Outcomes
The ultimate goal of hiring a consultant is actionable improvements that enhance compliance, efficiency, or performance. To achieve this:
- Define Clear Objectives
- Before engaging a consultant, outline your project scope, goals, budget, and desired outcomes. This clarity helps both parties align expectations.
- Insist on Detailed Proposals
- Request proposals that include timelines, deliverables, methodologies, and pricing structures. This ensures transparency and sets benchmarks for success.
- Collaborate Throughout the Process
- Involve your team in discussions with the consultant to ensure alignment on priorities and feasibility of recommendations.
- Monitor Implementation
- Establish metrics to track progress against the consultant’s recommendations (e.g., compliance rates, operational efficiency improvements).