Document change control has a core set of requirements for managing critical information throughout its lifecycle. These requirements encompass:
- Approval of documents based on fit-for-purpose and fit-for-use before issuance
- Review and document updates as needed (including reapprovals)
- Managing changes and revision status
- Ensuring availability of current versions
- Maintaining document legibility and identification
- Controlling distribution of external documents
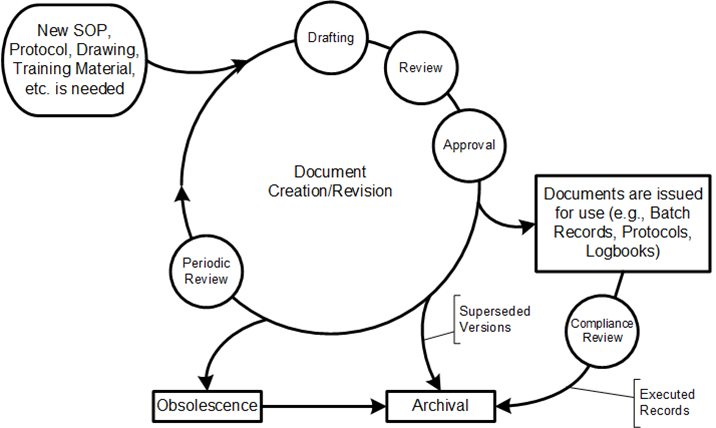
This lifecycle usually has three critical dates associated with approval:
- Approval Date: When designated authorities have reviewed and approved the document
- Issuance Date: When the document is released into the document management system
- Effective Date: When the document officially takes effect and must be followed
These dates are dependent on the type of document and can change as a result of workflow decisions.
| Type of Document | Approval Date | Issuance date | Effective Date |
| Functional | Date Approved by final approver (sequential or parallel) | Date Training Made Available | End of Training Period |
| Record | Date Approved by final approver (sequential or parallel) | Usually automated to be same as Date Approved | Usually same as Date Approved |
| Report | Date Approved by final approver (sequential or parallel) | Usually automated to be same as Date Approved | Usually same as Date Approved |
At the heart of the difference between these three days is the question of implementation and the Effective Date. At its core, the effective date is the date on which the requirements, instructions, or obligations in a document become binding for all affected parties. In the context of GxP document management, this represents the moment when:
- Previous versions of the document are officially superseded
- All operations must follow the new procedures outlined in the document
- Training on the new procedures must be completed
- Compliance audits will use the new document as their reference standard
Why Training Periods Matter in GxP Environments
One of the most frequently overlooked aspects of document management is the implementation period between document approval and its effective date. This period serves a critical purpose: ensuring that all affected personnel understand the document’s content and can execute its requirements correctly before it becomes binding.
In order to implement a new process change in a compliant manner, people must be trained in the new procedure before the document becomes effective. This fundamental principle ensures that by the time a new process goes “live,” everyone is prepared to perform the revised activity correctly and training records have been completed. Without this preparation period, organizations risk introducing non-compliance at the very moment they attempt to improve quality.
The implementation period bridges the gap between formal approval and practical application, addressing the human element of quality systems that automated solutions alone cannot solve.
Selecting Appropriate Implementation Periods
When configuring document change control systems, organizations must establish clear guidelines for determining implementation periods. The most effective approach is to build this determination into the change control workflow itself.
Several factors should influence the selection of implementation periods:
- Urgency: In cases of immediate risk to patient safety or product quality, implementation periods may be compressed while still ensuring adequate training.
- Risk Assessment: Higher-risk changes typically require more extensive training and therefore longer implementation periods.
- Operational Impact: Changes affecting critical operations may need carefully staged implementation.
- Training Complexity: Documents requiring hands-on training necessitate longer periods than read-only procedures.
- Resource Availability: Consider the availability of trainers and affected personnel
Determining Appropriate Training Periods
The time required for training should be determined during the impact assessment phase of the change approval process. This assessment should consider:
- The number of people requiring training
- The complexity of the procedural changes
- The type of training required (read-only versus observed assessment)
- Operational constraints (shift patterns, production schedules)
Many organizations standardize on a default period (typically two weeks), but the most effective approach tailors the implementation period to each document’s specific requirements. For critical processes with many stakeholders, longer periods may be necessary, while simple updates affecting few staff might require only minimal time.
Consider this scenario: Your facility operates two shifts with 70 people during the day and 30 at night. An updated SOP requires all operators to complete not just read-only training but also a one-hour classroom assessment. If manufacturing schedules permit only 10 operators per shift to attend training, you would need a minimum of 7 days before the document becomes effective. Without this calculated implementation period, every operator would instantly become non-compliant when the new procedure takes effect.
Early Use of Documents
The distinction between a procedure’s approval date and its effective date serves a critical purpose. This gap allows for proper training and implementation before the procedure becomes binding. However, there are specific circumstances when personnel might appropriately use a procedure they’ve been trained on before its official effective date.
1. Urgent Safety or Quality Concerns
When there is an immediate risk to patient safety or product quality, the time between approval and effectiveness may be compressed. For these cases there should be a mechanism to move up the effective date.
In such cases, the organization should prioritize training and implementation while still maintaining proper documentation of the accelerated timeline.
2. During Implementation Period for Training Purposes
The implementation period itself is designed to allow for training and controlled introduction of the new procedure. During this time, a limited number of trained personnel may need to use the new procedure to:
- Train others on the new requirements
- Test the procedure in a controlled environment
- Prepare systems and equipment for the full implementation
These are all tasks that should be captured in the change control.
3. For Qualification and Validation Activities
During qualification protocol execution, procedures that have been approved but are not yet effective may be used under controlled conditions to validate systems, equipment, or processes. These activities typically occur before full implementation and are carefully documented to demonstrate compliance. Again these are captured in the change control and appropriate validation plan.
In some regulatory contexts, such as IRB approvals in clinical research, there are provisions for “approval with conditions” where certain activities may proceed before all requirements are finalized2. While not directly analogous to procedure implementation, this demonstrates regulatory recognition of staged implementation approaches.
Required Controls When Using Pre-Effective Procedures
If an organization determines it necessary to use an approved but not yet effective procedure, the following controls should be in place:
- Documented Risk Assessment: A risk assessment should be conducted and documented to justify the early use of the procedure, especially considering potential impacts on product quality, data integrity, or patient safety.
- Authorization: Special authorization from management and quality assurance should be obtained and documented.
- Verification of Training: Evidence must be available confirming that the individuals using the procedure have been properly trained and assessed on the new requirements.
What About Parallel Compliance with Current Effective Procedures?
In all cases, the currently effective procedure must still be followed until the new procedure’s effective date. However there are changes, usually as a result of process improvement, usually in knowledge work processes where it is possible to use parts of the new procedure. For example, the new version of the deviation procedure adds additional requirements for assessing the deviation, or a new risk management tool is rolled out. In these cases you can meet the new compliance path without violating the current compliance path. The organization should demonstrate how both compliance paths are being maintained.
In cases where the new compliance path does not contain the old, but instead offers a new pathway, it is critical to maintain one way of work-as-prescribed and the effective date is a solid line.
Organizations should remember that the implementation period exists to ensure a smooth, compliant transition between procedures. Any exception to this standard approach should be carefully considered, well-justified, and thoroughly documented to maintain GxP compliance and minimize regulatory risk.
