Just finished up the 2022 ISPE Aseptic Conference, and here are a few thoughts.
EU GMP Annex 1 expected in later half of the year
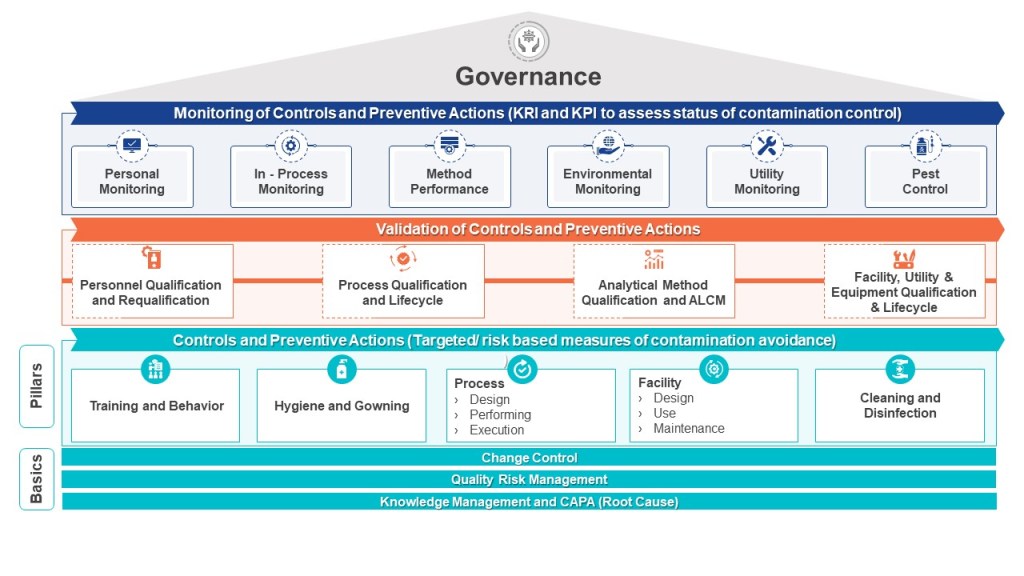
Paul Gustafson, chair of the Pharmaceutical Inspection Co-operation Scheme (PIC/S) and a senior corporate regulatory compliance and enforcement advisor with Health Canada, stated that the plan was to issue the widely anticipated Annex 1 in mid-year 2022. He repeatedly said July to September so that is interesting news and start getting your contamination control strategies going. There will be a one-year period before in force, with 2 years on some of the lyophilizer requirements.
For those keeping track, it retains the provision calling for testing filters used in the sterilization process, pre-use, post-sterilization integrity testing (PUPSIT). The PUPSIT provision “has driven a substantial amount of discussion and has resulted in a number of papers being drafted,” said Gustafson. This was a very gracious understatement, and I have to admit I really admired his Canadian humor.
FDA continues to evaluate COVID inspection measures
Alonza Cruse, Director of the Office of Pharmaceutical Quality Operations at FDA/ORA did a thorough job going through the COVID measures of Remote Regulatory Assessments and Remote Interactive Evaluations and discussed how the agency was in the process of learning how best to do things going forward.
He also clearly state how they were continuing to get back to normal inspections and discussed new personnel in foreign offices, such as India.
Highlights from Panels
One of my favorite panels was Jo Ann Jacobs and Kara Vogt speaking on “Building Resiliency into Single-Use-Technology Systems” They laid out some good work they are doing as part of a startup to design good functional equivalency and supplier management, obviously learning from PPAP and similar measures. Quite well done. While it leans heavily into my own practice around functional equivalency it was good to see such a rock-solid implementation, and I felt like I learned a few good ideas.
I spoke on Contamination Control, Risk Management and the Quality Management System, having a blast doing so. I was followed by Christa Myers who spoke on “Contamination Control Strategy: From Annex 1 Draft Requirements to Implementation in Practice.” We made a good duo and between the two I hope participants got a real solid idea on how to do this contamination control strategy effectively.
I learned a lot about robotics and isolators.
Still a big fan of ISPE’s Women in Pharma.