One of the many fascinating items in the recent Warning Letter to Sanofi is the FDA’s direction to provide a plan to perform “timely technological upgrades to the equipment/facility infrastructure.” This point drives home the point that staying current with technological advancements is crucial for maintaining compliance, improving efficiency, and ensuring product quality. Yet, I think it is fair to say we rarely see it this bluntly put as a requirement.
One of the many reasons this Warning Letter stands out is that this is (as far as I can tell) the same facility that won the ISPE’s Facility of the Year award in 2020. This means it is still a pretty new facility, and since it is one of the templates that many single-use biotech manufacturing facilities are based on, we had best pay attention. If a failure to maintain a state-of-the-art facility can contribute to this sort of Warning Letter, then many companies had best be paying close attention. There is a lot to unpack and learn here.
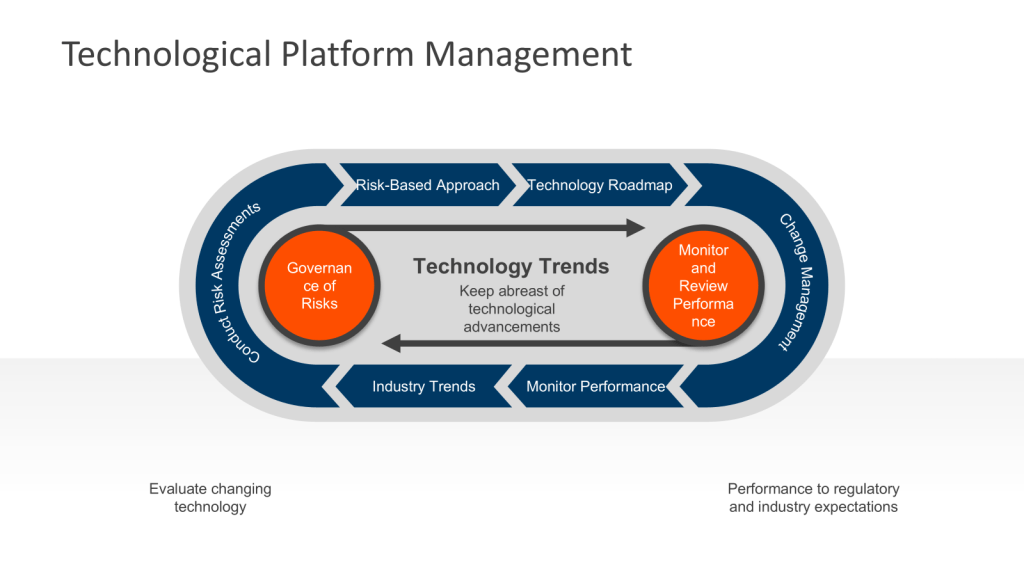
Establishing an Ongoing Technology Platform Process
To meet regulatory requirements and industry standards, facilities should implement a systematic approach to technological upgrades.
1. Conduct Regular Assessments
At least annually, perform comprehensive evaluations of your facility’s equipment, systems, and processes. This assessment should include:
- Review of equipment performance and maintenance, including equipment effectiveness
- Analysis of deviation reports and quality issues
- Evaluation of current technologies against emerging industry standards
- Assessment of facility design and layout for potential improvements
This should be captured as part of the FUSE metrics plan and appropriately evaluated as part of quality governance.
2. Stay Informed on Industry Trends
Keep abreast of technological advancements in biotech manufacturing at minimum by:
- Attending industry conferences and workshops
- Participating in working groups for key consensus standard writers, such as ISPE and ASTM
- Subscribing to relevant publications and regulatory updates
- Engaging with equipment vendors and technology providers
3. Develop a Risk-Based Approach
Prioritize upgrades based on their potential impact on product quality, patient safety, and regulatory compliance. Utilize living risk assessments to get a sense of where issues are developing. These should be the evolution of the risk management that built the facility.
4. Create a Technology Roadmap
Develop a long-term plan for implementing upgrades, considering:
- Budget constraints and return on investment
- Regulatory timelines for submissions and approvals
- Production schedules and potential downtime
- Integration with existing systems and processes
5. Implement Change Management Procedures
Ensure there is a robust change management process in place to ensure that upgrades are implemented safely and effectively. This should include:
- Detailed documentation of proposed changes
- Impact assessments on product quality and regulatory compliance
- Appropriate verification for new and changed equipment and systems
- Training programs for personnel
6. Appropriate Verification – Commissioning, Qualification and Validation
Conduct thorough verification activities to demonstrate that the upgraded equipment or systems meet predetermined specifications and regulatory requirements.
7. Monitor and Review Performance
Continuously monitor the performance of upgraded systems and equipment to ensure they meet expectations and comply with cGMP requirements. Conduct periodic reviews to identify any necessary adjustments or further improvements. This is all part of Stage 3 of the FDA’s process validation model focusing on ongoing assurance that the process remains in a state of control during routine commercial manufacture. This stage is designed to:
- Anticipate and prevent issues before they occur
- Detect unplanned deviations from the process
- Identify and correct problems
Leveraging Advanced Technologies
To stay ahead of regulatory expectations and industry trends, consider incorporating advanced technologies into your upgrade plans:
- Single-Use Systems (SUS): Implement disposable components to reduce cleaning and validation requirements while improving flexibility.
- Modern Microbial Methods (MMM): Implement advanced techniques used in microbiology that offer significant advantages over traditional culture-based methods
- Process Analytical Technology (PAT): Integrate real-time monitoring and control systems to enhance product quality and process understanding.
- Data Analytics and Artificial Intelligence: Implement advanced data analysis tools to identify trends, predict maintenance needs, and optimize processes.
Conclusion
Maintaining a state-of-the-art biotech facility requires a proactive and systematic approach to technological upgrades. By establishing an ongoing process for identifying and implementing improvements, facilities can ensure compliance with FDA requirements, align with industry standards, and stay competitive in the rapidly evolving biotech landscape.
Remember that the goal is not just to meet current regulatory expectations but to anticipate future requirements and position your facility at the forefront of biotech manufacturing excellence. By following this comprehensive approach and staying informed on industry developments, you can create a robust, flexible, and compliant manufacturing environment that supports the production of high-quality biopharmaceutical products.
