There have been a lot of changes in the way pharma thinks of analytical lifecycles in the last few years. With changes in technology, new product modalities, ICH Q2(R2) and ICH Q14 being released in November 2023, and USP <1220> in 2022, it is fair to say we are all catching up with our analytical lifecycle programs.
Let’s discuss what I think are the four pivotal documents that provide direction.
ICH Q2(R2) and ICH Q14
ICH Q2(R2) and ICH Q14 are complementary guidelines that provide a comprehensive framework for the development, validation, and lifecycle management of analytical procedures used in the pharmaceutical industry.
ICH Q14 describes the scientific principles and risk-based approaches for developing and maintaining suitable analytical procedures throughout their lifecycle. It outlines the key elements and considerations for analytical procedure development, including:
- Defining an Analytical Target Profile (ATP)
- Knowledge management and risk assessment
- Evaluating robustness and parameter ranges
- Establishing an Analytical Procedure Control Strategy
- Lifecycle management and post-approval changes
- Multivariate analytical procedures
- Real-time release testing
On the other hand, ICH Q2(R2) provides specific guidance on validating analytical procedures to demonstrate their suitability for the intended use. It covers various validation tests, methodologies, and evaluation criteria, such as:
- Specificity/selectivity
- Working range
- Accuracy and precision
- Robustness
- Stability-indicating properties
- Multivariate analytical procedures
In summary, ICH Q14 establishes the overarching principles and approaches for analytical procedure development. At the same time, ICH Q2(R2) focuses on the validation aspects to ensure the analytical procedures are fit for purpose and meet quality requirements throughout their lifecycle. The two guidelines are intended to be applied together, with ICH Q14 providing the framework for development and ICH Q2(R2) specifying the validation requirements.
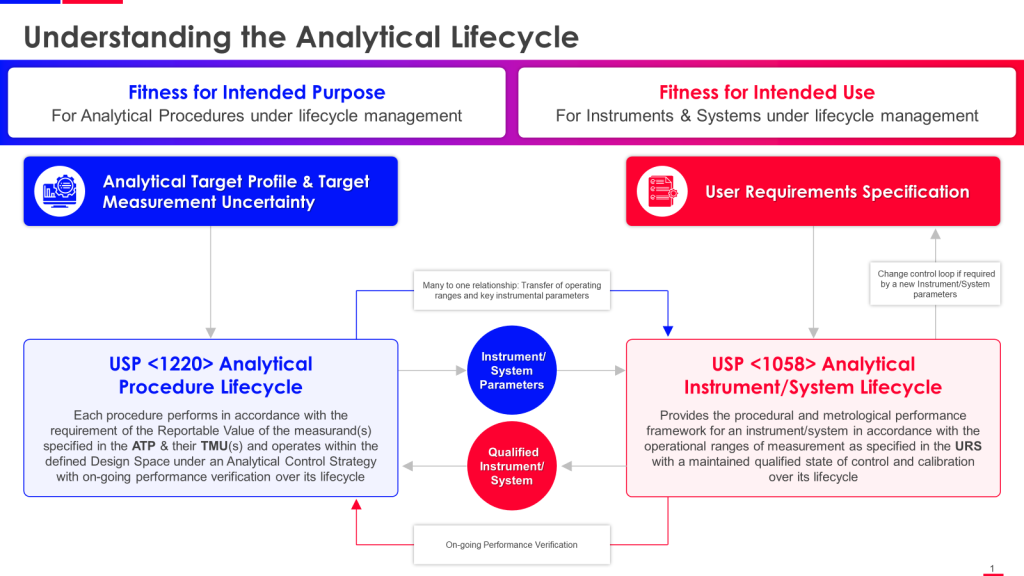
USP <1220> Analytical Procedure Lifecycle and USP <1058> Analytical Instrument Qualification
USP <1220> Analytical Procedure Lifecycle and USP <1058> Analytical Instrument Qualification are closely connected and complementary guidelines that provide a comprehensive framework for ensuring data integrity and quality in analytical procedures throughout their lifecycle.
The key connections between USP <1220> and USP <1058> are:
- USP <1220> establishes the principles and requirements for managing the entire lifecycle of analytical procedures, from procedure design and development to retirement. It emphasizes the importance of defining an Analytical Target Profile (ATP) and implementing an Analytical Procedure Control Strategy.
- USP <1058> focuses explicitly on the qualification of analytical instruments that execute analytical procedures. It outlines the requirements for ensuring instruments are suitable for their intended use through proper qualification (Design, Installation, Operational, and Performance Qualification).
- The instrument qualification activities described in USP <1058> are critical to the overall Analytical Procedure Control Strategy outlined in USP <1220>. Proper instrument qualification as per <1058> helps ensure the quality and integrity of data generated by analytical procedures throughout their lifecycle.
- Both guidelines stress the importance of defining user requirements (ATP in <1220> and User Requirements Specification in <1058>) as the basis for procedure development and instrument qualification activities.
- USP <1220> requires ongoing monitoring and periodic requalification of analytical procedures, which includes re-evaluating the suitability of the analytical instruments used, as described in the Performance Qualification section of <1058>.
USP <1220> provides the overarching framework for holistically managing analytical procedures. USP <1058> focuses on ensuring the instruments used to execute those procedures are properly qualified and suitable for their intended use. The two guidelines work together to maintain data integrity and quality across the entire analytical lifecycle.
Complementary Approaches
USP <1220> Analytical Procedure Lifecycle is closely related to and complements the ICH Q2(R2) and ICH Q14 guidelines.
- USP <1220> aligns with the principles outlined in ICH Q14 for managing the entire lifecycle of analytical procedures, from design and development to retirement. Both emphasize defining an Analytical Target Profile and implementing an Analytical Procedure Control Strategy.
- The validation activities described in ICH Q2(R2), such as evaluating specificity, accuracy, precision, and robustness, are critical components of the Analytical Procedure Control Strategy required by USP <1220>.
- USP <1220> requires ongoing monitoring and periodic requalification of analytical procedures, which aligns with the lifecycle management approach promoted in ICH Q14 and the validation during the lifecycle section in Q2(R2).
- All these guidelines stress the importance of knowledge management, risk management, and a science/risk-based approach throughout the analytical procedure lifecycle.
- The instrument qualification requirements outlined in USP <1058> are an integral part of the overall Analytical Procedure Control Strategy described in USP <1220>, ensuring instruments are suitable as per ICH Q2(R2) validation principles.
In essence, USP <1220> provides a comprehensive framework for analytical procedure lifecycle management that incorporates and operationalizes the scientific principles and validation activities detailed in the ICH Q14 and Q2(R2) guidelines, while USP <1058> provides the roadmap for instrument qualification. These four documents establish harmonized best practices for analytical procedures from development through retirement.

One thought on “The State of the Analytical Lifecycle”