To complete a thought on community of practices I did this weekend with “A CoP is Collaborative Learning, not Lecture” and “How I would Organize a Meeting of a CoP” I’m now going to build, from the group up, a collaborative learning event I would love to organize.
A little caveat: I really burnt out on professional obligations last year and have just started to peak my head out. So, it may be a little harder to turn this mad scientist dream into a reality. However, I think it is worth putting out as a thought experiment.
Theme and Scope
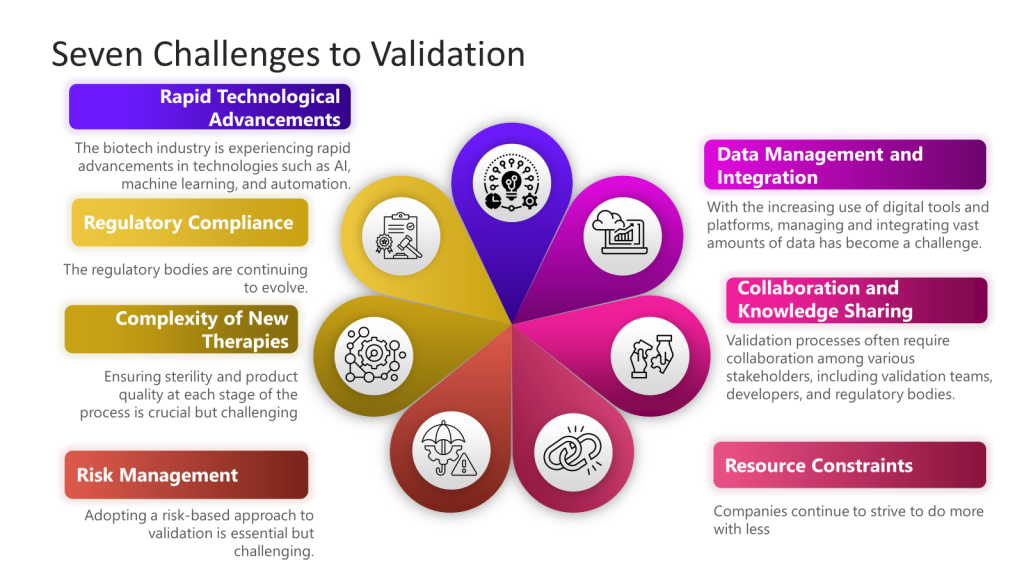
I’ve written a bit about the challenges to quality, and these challenges provide a framework for much of what I think and write about.
More specifically drawing from the “Challenges in Validation” focusing on the challenges of navigating a complex validation landscape characterized by rapid technological advancements, evolving regulatory standards, and the development of novel therapies.
This event would ask, “How do we rise to the challenges of validation in the next decade, leveraging technology and a risk management approach and drawing from the best practices of ASTM E2500, GAMP5, and others to meet and exceed changing regulatory requirements.”
Intended Audience
I go to events, and there are a lot of quality people, OR risk management people, OR computer systems (IT and Q) people, OR engineers, OR analytical method folks, OR process development people. Rarely do I see an event that looks at the whole picture. And rarely do I get to attend an event where we are sharing and blurring the lines between the various silos. So let us break down the silos and invite quality, IT, engineers, and process development individuals involved in the full spectrum of pharmaceutical (and possibly medtech) validation.

This holistic event is meant to blend boundaries, share best practices, challenge ourselves, and look across the entire validation lifecycle.
Structure
Opening/Networking (1 hour)
As people arrive, they go right into a poster event. These posters are each for a specific methodology/approach of ASTM E2500, ISPE Baseline Guides, FDA’s Guidance for Process Validation: General Principles and Practices, ICH’s QbD approach, and GAMP5. Maybe some other things.
These posters would each:
- Provide an overview of what it is and why it is important
- Overview of methodology
- What challenges it overcomes
- Lessons that can be applied
- Challenges/problems inherent in the approach
These posters would be fun to develop and take a good squad of experts.
After an hour of mingling, sharing, and baselining, we could move to the next step.
Fish Bowl Debate (45 minutes)
Having earlier selected a specific topic and a panel of experts, hold a fish bowl debate. This would be excellent as a mock-inspection, maybe of a really challenging topic. Great place to bring those inspectors in.
During a fish bowl, everyone not in the center is taking notes. I love a worksheet to help with this by providing things to look for to get the critical thinking going.
Future Workshop (1.5 hour)
- Introduce the activity (10 min)
- Ask participants to reflect on their present-day situation, write down all their negative experiences on sticky notes, and place them on the wall. (15 min)
- Invite participants to list uncertainties they face by asking, “In your/our operating environment, what factors are impossible to predict or control their direction?” (5 min).
- Prioritize the most critical factors by asking, “Which factors threaten your/our ability to operate successfully?” (10 min)
- Based on the group’s history and experience, select the two most critical and most uncertain (X and Y). (5 min)
- Create a grid with two axes—X & Y—with a “more of <— —> less of” continuum to represent the factor on each axis. For example, suppose new modalities are a critically uncertain factor for the X-axis. In that case, one end of the X-axis is many new modalities, and the other is no new modalities. Repeat for the Y factor and axis. For instance, if patent protection is a critical factor, one end of the Y axis is strong patent protection, and the other has no patent protection. Four quadrants are created. (5 min)
- Break into four groups, and each group creatively names and writes a thumbnail scenario for one of the quadrants. (10 min)
- The four groups share their scenarios briefly. 2 min. each
- Participants fantasize about the desired future situation. How would the ideal situation be for them? At this stage, there are no limitations; everything is possible. Write on stick notes and apply them to the most likely quadrant. (10 minutes)
- Do a n/3 activity to find the top ideas (enough for groups of 4-5 each) (3 min)
- Explain the next activity (2 min)
Lunch (1 hour)
Open Space Solution (1 hour)
For each top idea, the participants vote with their feet and go to develop the concept. Each group is looking to come up with the challenge solved, a tool/methodology, and an example.
Review the Results of the Open Space Solutions (1 hour)
Each team presents for 5-8 minutes.
1-2-4-All (20 minutes)
- Silent self-reflection by individuals on the shared challenge, framed as a question “What opportunities do YOU see for making progress on this challenge? How would you handle this situation? What ideas or actions do you recommend?” (1 min)
- Generate ideas in pairs, building on ideas from self-reflection. (2 min)
- Share and develop ideas from your pair in foursomes (notice similarities and differences).( 4 min)
- Ask, “What is one idea that stood out in your conversation?” Each group shares one important idea with all (15 min)
Closing Commitment (5 min)
Where will this live? What comes next? Make a commitment to follow up electronically.
Networking
Spend an hour or so with drinks and food and discuss everything. Never enough socialization.