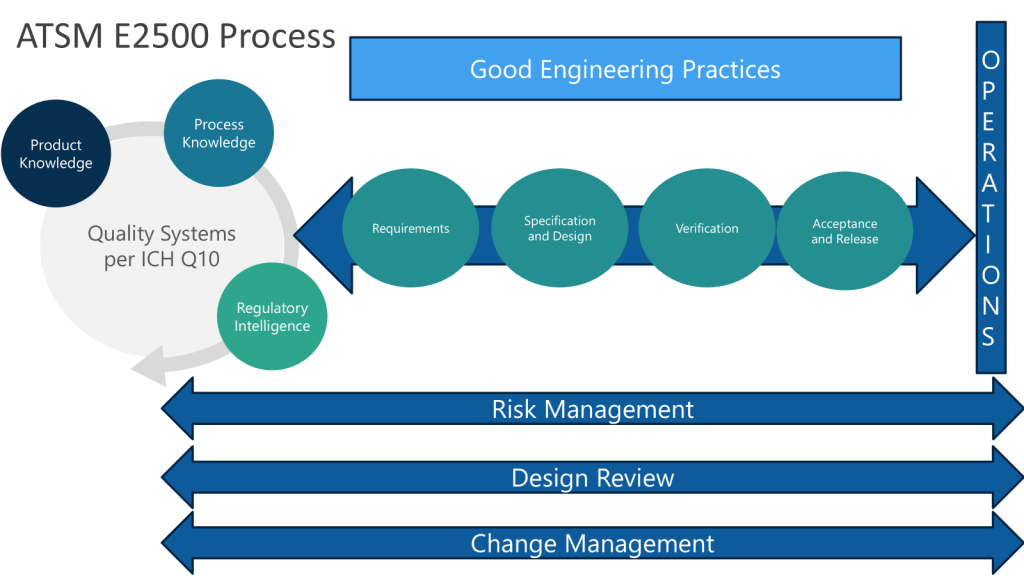
ASTM E2500 recognizes that Good Engineering Practices (GEP) are essential for pharmaceutical companies to ensure the consistent and reliable design, delivery, and operation of engineered systems in a manner suitable for their intended purpose.
Key Elements of Good Engineering Practices
- Risk Management: Applying systematic processes to identify, assess, and control risks throughout the lifecycle of engineered systems. This includes quality risk management focused on product quality and patient safety.
- Cost Management: Estimating, budgeting, monitoring and controlling costs for engineering projects and operations. This helps ensure projects deliver value and stay within budget constraints.
- Organization and Control: Establishing clear organizational structures, roles and responsibilities for engineering activities. Implementing monitoring and control mechanisms to track performance.
- Innovation and Continual Improvement: Fostering a culture of innovation and continuous improvement in engineering processes and systems.
- Lifecycle Management: Applying consistent processes for change management, issue management, and document control throughout a system’s lifecycle from design to decommissioning.
- Project Management: Following structured approaches for planning, executing and controlling engineering projects.
- Design Practices: Applying systematic processes for requirements definition, design development, review and qualification.
- Operational Support: Implementing asset management, calibration, maintenance and other practices to support systems during routine operations.
Key Steps for Implementation
- Develop and document GEP policies, procedures and standards tailored to the company’s needs
- Establish an Engineering Quality Process (EQP) to link GEP to the overall Pharmaceutical Quality System
- Provide training on GEP principles and procedures to engineering staff
- Implement risk-based approaches to focus efforts on critical systems and processes
- Use structured project management methodologies for capital projects
- Apply change control and issue management processes consistently
- Maintain engineering documentation systems with appropriate controls
- Conduct periodic audits and reviews of GEP implementation
- Foster a culture of quality and continuous improvement in engineering
- Ensure appropriate interfaces between engineering and quality/regulatory functions
The key is to develop a systematic, risk-based approach to GEP that is appropriate for the company’s size, products and operations. When properly implemented, GEP provides a foundation for regulatory compliance, operational efficiency and product quality in pharmaceutical manufacturing.
Invest in a Living, Breathing Engineering Quality Process (EQP)
The EQP establishes the formal connection between GEP and the Pharmaceutical Quality System it resides within, serving as the boundary between Quality oversight and engineering activities, particularly for implementing Quality Risk Management (QRM) based integrated Commissioning and Qualification (C&Q).
It should also provide an interface between engineering activities and other systems like business operations, health/safety/environment, or other site quality systems.
Based on the information provided in the document, here is a suggested table of contents for an Engineering Quality Process (EQP):
Table of Contents – Engineering Quality Process (EQP)
- Introduction
1.1 Purpose
1.2 Scope
1.3 Definitions - Application and Context
2.1 Relationship to Pharmaceutical Quality System (PQS)
2.2 Relationship to Good Engineering Practice (GEP)
2.3 Interface with Quality Risk Management (QRM) - EQP Elements
3.1 Policies and Procedures for the Asset Lifecycle and GEPs
3.2 Risk Assessment
3.3 Change Management
3.4 Document Control
3.5 Training
3.6 Auditing - Deliverables
4.1 GEP Documentation
4.2 Risk Assessments
4.3 Change Records
4.4 Training Records
4.5 Audit Reports - Roles and Responsibilities
5.1 Engineering
5.2 Quality
5.3 Operations
5.4 Other Stakeholders - EQP Implementation
6.1 Establishing the EQP
6.2 Maintaining the EQP
6.3 Continuous Improvement - References
- Appendices

6 thoughts on “Good Engineering Practices Under ASTM E2500”