When engaged in regulatory/quality intelligence you should have a program in place to monitor for non-compliance reports, evaluate the internal quality system against those reports, and take appropriate preventative action. This is a fundamental risk management activity.
I tend to post about interesting 483s and Warning Letters fairly often, but one thing you won’t see me do is often delve deep into non-compliance reports from countries like India and China. For a manufacturer based in the US, this can often be a fair bit of noise, as the general state of the GMPs is different between the regions. The level of quality intelligence valuable to me if I was in India is different when I only support US and European sites.
I tend to follow a mode that looks like this:
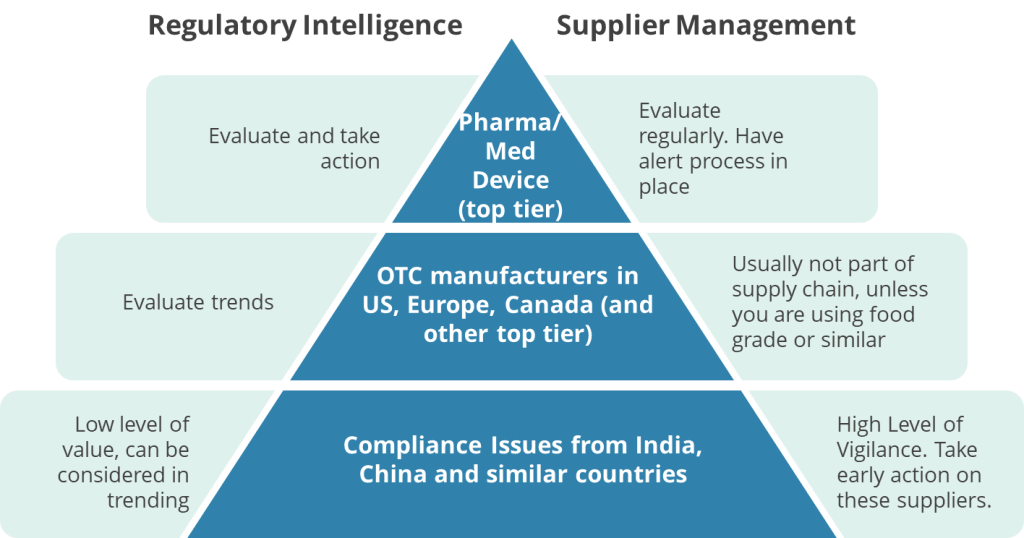
I apply two different urgency levels between regulatory intelligence (preventive action) and supplier management (ensuring baseline is compliant).
Focusing on regulatory intelligence, I ensure we evaluate each and every noncompliance report coming from pharma and medical device for companies in the US, Europe, Canada, Japan. Each one of those is evaluated to see if a similar issue could potentially be found.
OTC and similar manufacturers from those markets end up in the trending evaluation. Might not drive immediate action, but trends should.
Noncompliances from developing regions, like China and India I rarely give much thought to in regulatory intelligence. They will end up in trending, such as a yearly look at 483s, but in themselves there is usually little that is actionable.
As a consumer, there is a different, and unfortunately, worse story.

2 thoughts on “Not all Non-Compliance Reports are Equal”