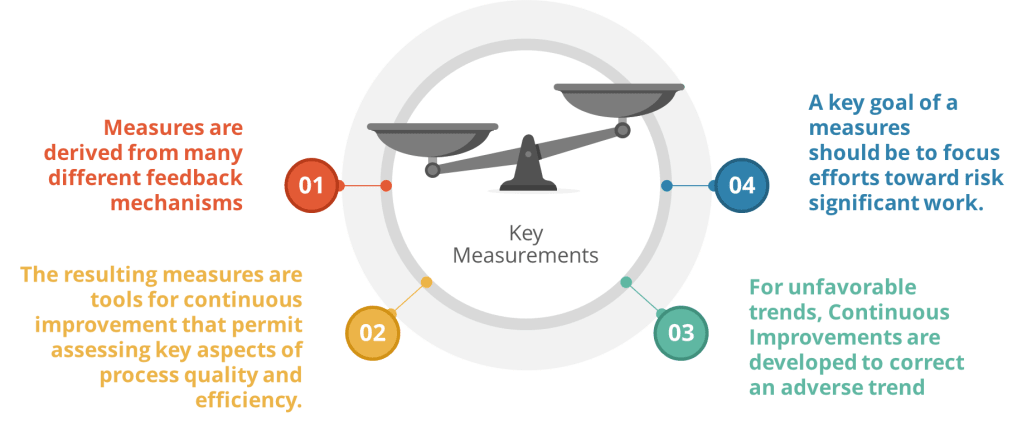
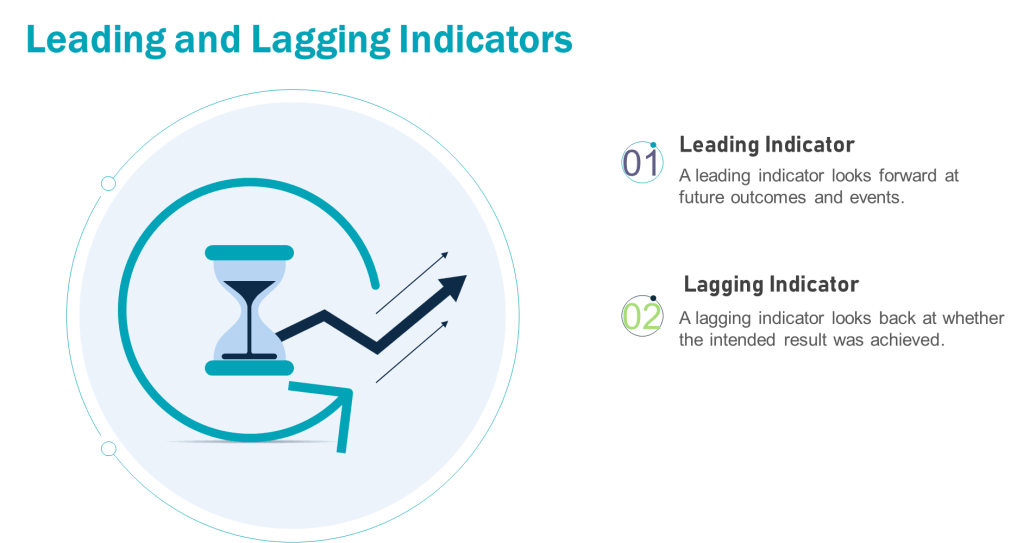
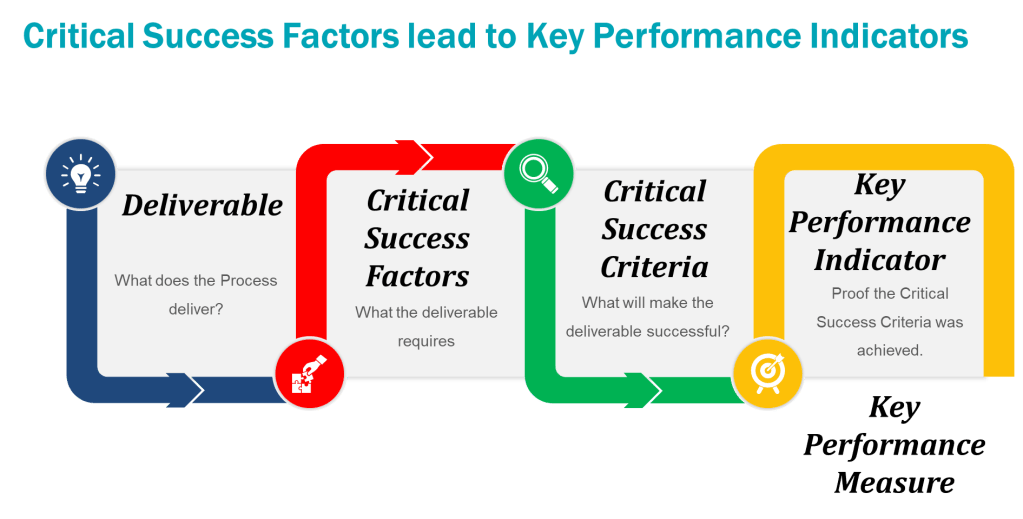
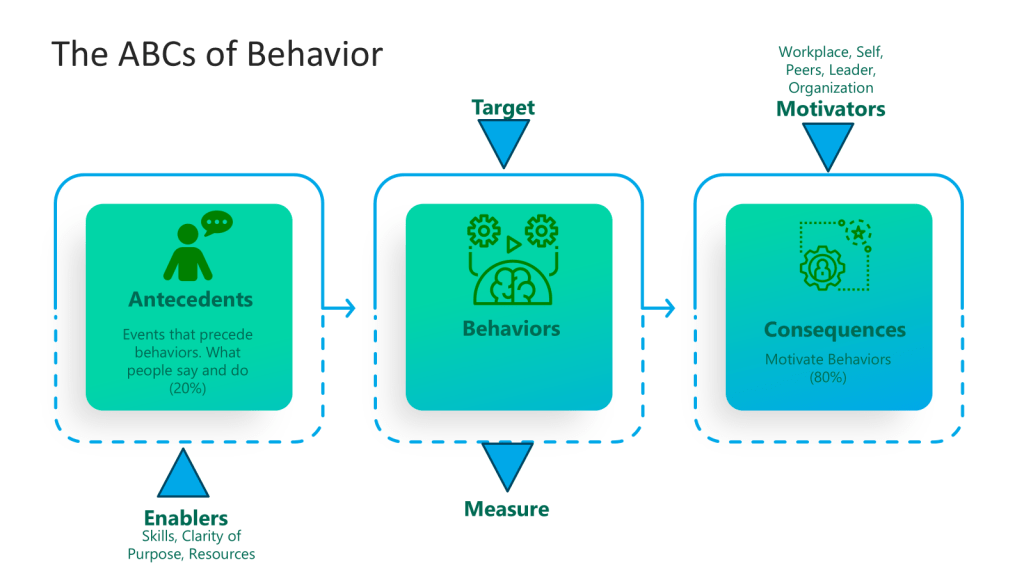
When thinking about the training program you can add the Kilpatrick model to the mix and build from there. This allows a view across the training system to drive for an effective training program.
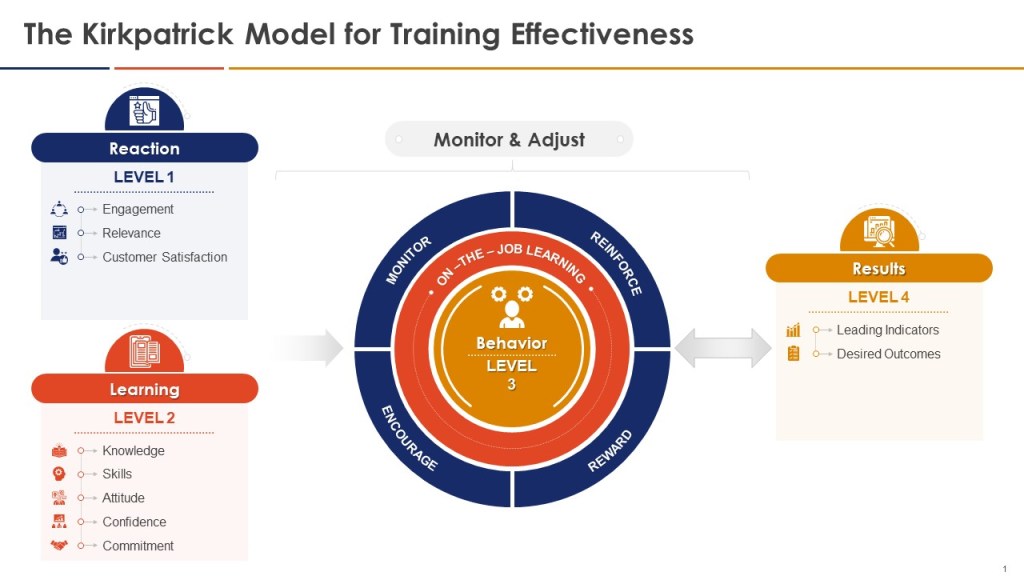
GMP Training Metrics Framework Aligned with Kirkpatrick’s Model
| Kirkpatrick Level | Category | Metric Type | Example | Purpose | Data Source | Regulatory Alignment |
|---|---|---|---|---|---|---|
| Level 1: Reaction | KPI | Leading | % Training Satisfaction Surveys Completed | Measures engagement and perceived relevance of GMP training | LMS (Learning Management System) | ICH Q10 Section 2.7 (Training Effectiveness) |
| KRI | Leading | % Surveys with Negative Feedback (<70%) | Identifies risk of disengagement or poor training design | Survey Tools | FDA Quality Metrics Reporting (2025 Draft) | |
| KBI | Leading | Participation in Post-Training Feedback | Encourages proactive communication about training gaps | Attendance Logs | EU GMP Chapter 2 (Personnel Training) | |
| Level 2: Learning | KPI | Leading | Pre/Post-Training Quiz Pass Rate (≥90%) | Validates knowledge retention of GMP principles | Assessment Software | 21 CFR 211.25 (Training Requirements) |
| KRI | Leading | % Trainees Requiring Remediation (>15%) | Predicts future compliance risks due to knowledge gaps | LMS Remediation Reports | FDA Warning Letters (Training Deficiencies) | |
| KBI | Lagging | Reduction in Knowledge Assessment Retakes | Validates long-term retention of GMP concepts | Training Records | ICH Q7 Section 2.12 (Training Documentation) | |
| Level 3: Behavior | KPI | Leading | Observed GMP Compliance Rate During Audits | Measures real-time application of training in daily workflows | Audit Checklists | FDA 21 CFR 211 (cGMP Compliance) |
| KRI | Leading | Near-Miss Reports Linked to Training Gaps | Identifies emerging behavioral risks before incidents occur | QMS (Quality Management System) | ISO 9001:2015 Clause 10.2 (Nonconformity) | |
| KBI | Leading | Frequency of Peer-to-Peer Knowledge Sharing | Encourages a culture of continuous learning and collaboration | Meeting Logs | ICH Q10 Section 3.2.3 (Knowledge Management) | |
| Level 4: Results | KPI | Lagging | % Reduction in Repeat Deviations Post-Training | Quantifies training’s impact on operational quality | Deviation Management Systems | FDA Quality Metrics (Batch Rejection Rate) |
| KRI | Lagging | Audit Findings Related to Training Effectiveness | Reflects systemic training failures impacting compliance | Regulatory Audit Reports | EU GMP Annex 15 (Qualification & Validation) | |
| KBI | Lagging | Employee Turnover | Assesses cultural impact of training on staff retention | HR Records | ICH Q10 Section 1.5 (Management Responsibility) |
Kirkpatrick Model Integration
- Level 1 (Reaction):
- Leading KPI: Track survey completion to ensure trainees perceive value in GMP content.
- Leading KRI: Flag facilities with >30% negative feedback for immediate remediation .
- Level 2 (Learning):
- Leading KPI: Require ≥90% quiz pass rates for high-risk roles (e.g., aseptic operators) .
- Lagging KBI: Retake rates >20% trigger refresher courses under EU GMP Chapter 3 .
- Level 3 (Behavior):
- Leading KPI: <95% compliance during audits mandates retraining per 21 CFR 211.25 .
- Leading KRI: >5 near-misses/month linked to training gaps violates FDA’s “state of control” .
- Level 4 (Results):
- Lagging KPI: <10% reduction in deviations triggers CAPA under ICH Q10 Section 4.3 .
- Lagging KRI: Audit findings >3/year require FDA-mandated QMS reviews .
Regulatory & Strategic Alignment
- FDA Quality Metrics: Level 4 KPIs (e.g., deviation reduction) align with FDA’s 2025 focus on “sustainable compliance” .
- ICH Q10: Level 3 KBIs (peer knowledge sharing) support “continual improvement of process performance” .
- EU GMP: Level 2 KRIs (remediation rates) enforce Annex 11’s electronic training documentation requirements .
By integrating Kirkpatrick’s levels with GMP training metrics, organizations bridge knowledge acquisition to measurable quality outcomes while meeting global regulatory expectations.