Commissioning, qualification, and validation are three distinct but interrelated processes in the pharmaceutical and biotechnology industries that ensure facilities, equipment, systems, and processes meet regulatory requirements and produce products of the desired quality. Here are the key differences:
Commissioning
- Commissioning is a systematic process of ensuring that equipment, systems, and facilities are designed, installed, and functioning according to operational and engineering requirements.
- It involves design reviews, installation verification, functional testing, and handover to operations.
- Commissioning primarily focuses on satisfying engineering requirements and does not have direct regulatory requirements.
Qualification
- Qualification is a regulated and documented process that demonstrates that equipment, systems, and facilities are installed correctly and operate as intended for their specific use.
- It applies only to equipment, systems, and utilities that directly or indirectly impact product quality and patient safety.
- Qualification activities include Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
- Qualification is focused on by regulatory authorities like the FDA and EMA to ensure compliance.
Validation
- Validation is a broader concept establishing documented evidence that a process consistently produces a product that meets its predetermined specifications and quality attributes.
- It encompasses the entire process lifecycle, including process design, qualification of equipment/systems, and continued process verification.
- Validation ensures that the equipment and systems are qualified and the entire process is controlled to produce the desired final product.
In summary, commissioning verifies engineering requirements, qualification demonstrates suitability for intended use, and validation provides a high degree of assurance that the process will consistently produce a quality product. These activities are interconnected, with commissioning often leveraged during qualification and qualification being a subset of the overall validation process.
FDA’s Framework for Process Validation
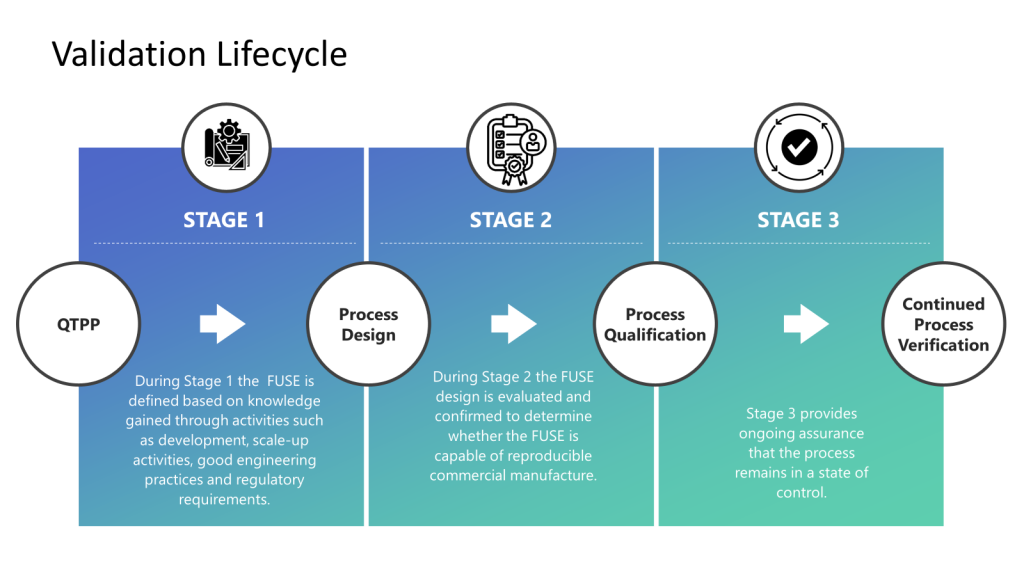
The FDA’s Process Validation Guidance is a core document outlining a lifecycle approach with outlines a lifecycle approach with three main stages:
Stage 1: Process Design
- Establish a process design based on knowledge gained through development and scale-up activities.
- Identify critical quality attributes (CQAs) and critical process parameters (CPPs) using risk assessment and multivariate studies like Design of Experiments (DoE).
- Develop a control strategy to ensure CQAs are met.
Stage 2: Process Qualification
- Evaluate the process design through facility, utility, and equipment qualification.
- Conduct performance qualification (PQ) by running production batches to confirm the process design has reproducible commercial manufacturing.
- Establish scientific evidence that the process meets all defined requirements and product specifications.
Stage 3: Continued Process Verification
- Maintain the validated status and monitor performance to ensure a state of control.
- Identify sources of variation and implement process improvements through an ongoing program.
- Conduct product quality reviews periodically to evaluate process performance.
The guidance emphasizes using a science and risk-based approach throughout the lifecycle, leveraging process understanding and knowledge gained from development through commercial production. Effective process validation requires good planning, documented evidence, and a robust quality system.

3 thoughts on “Commissioning, Qualification and Validation”