When I first joined my current company I spent a lot of time introducing myself. I’ve been here now a year, and there are new folks, new relationships and most important we are getting ready to change our way of working by introducing hybrid work.

As a leader it’s important to be honest in who you are and how you work. The best technique I’ve seen for this is a user manual, a quick way to express what works and what does not work for interacting with me.
Mine looks like this:
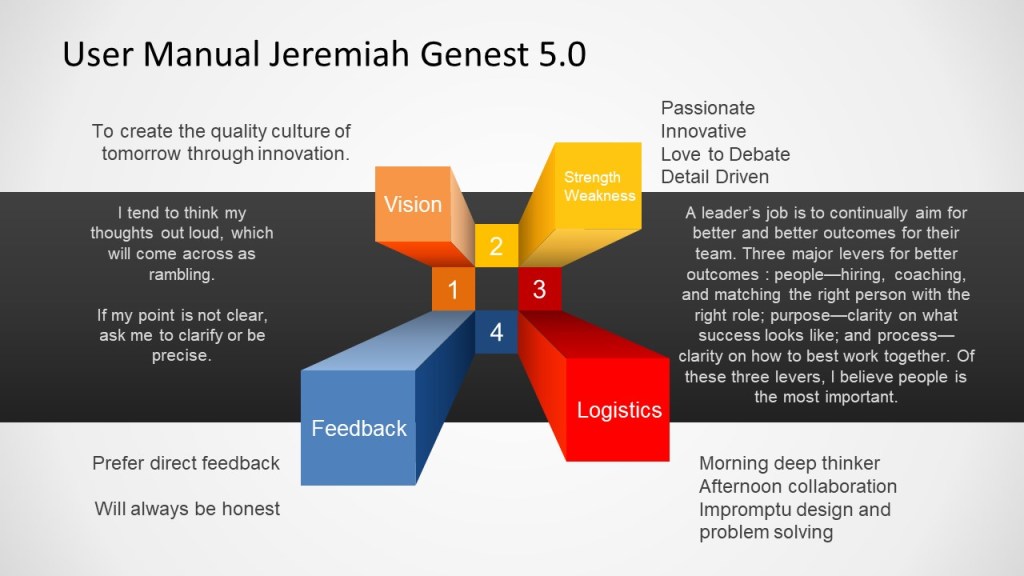
I’ll be updating this as part of my team establishing a new team governance charter.