I recently joined Just Evotec Biologics as the Senior Director of Global Quality Engineering and Validation. For a variety of reasons (just look at my past company on my LinkedIn bio and search the news to find one) it was a good time to move. I had decided that I wanted a position that was tied to an innovative manufacturing company and was deep in domain expertise. The combination of Just Evotec Biologics innovative technology aims and the ability to deep dive into one of my favorite topics was just too much to resist. Add to it the opportunity to work with a leader I deeply respected again and well, here I am. And feeling very good about it.
When I first started I met with the team and laid out my 30-60-90 day goals.
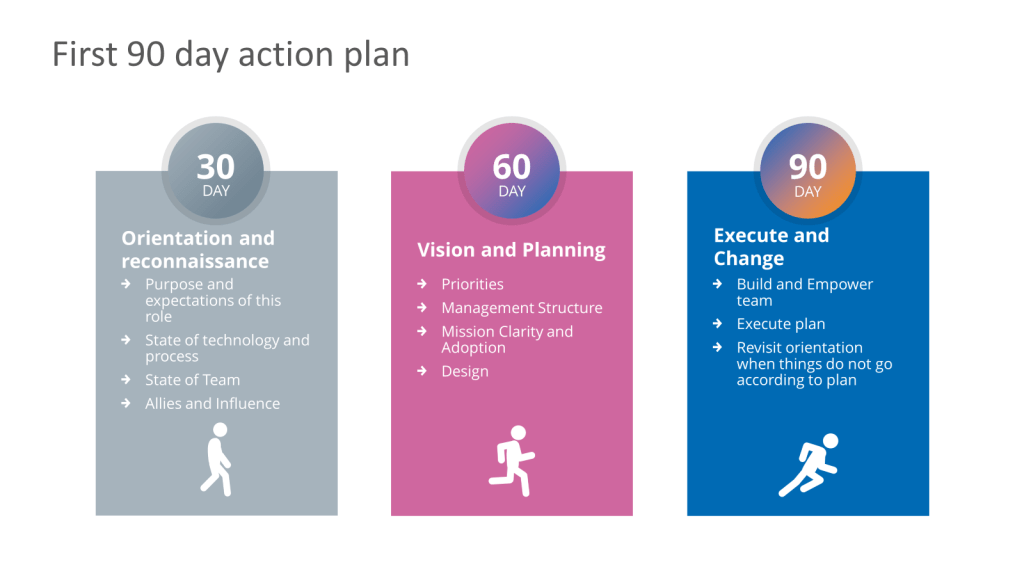
As well as talking a little about how I operate.
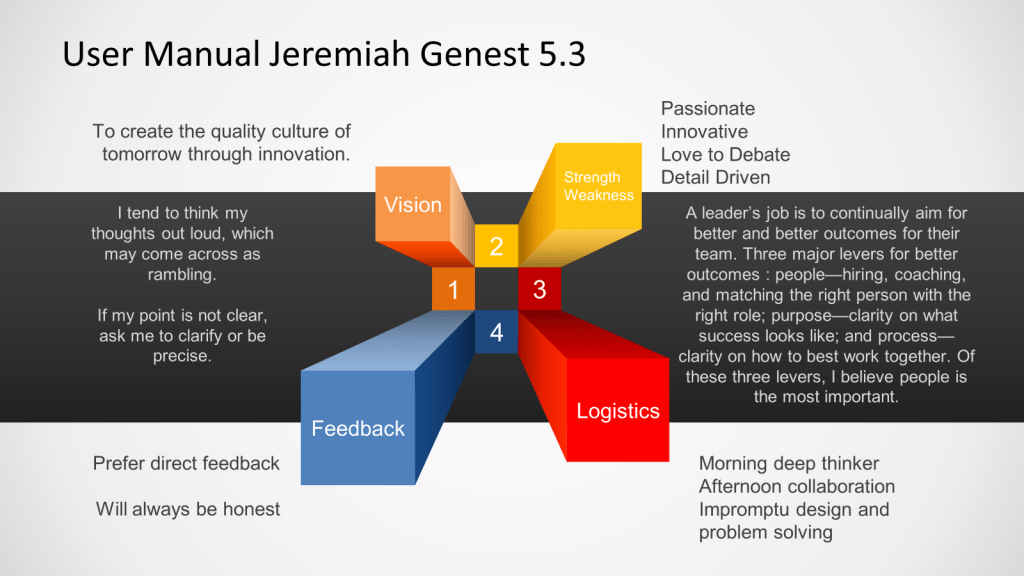
A big chunk of my time has been getting the lay-of-the-land institutionally. Setting some standards, doing gap assessments, figuring out what-is-what, and getting to know all my partners and stakeholders. For reasons of confidentiality, this post won’t be going deep on that.
What I do want to talk about is our team values and ways of working. I’ve been focused heavily on three areas with the team:
- Team Values
- Team Decision Making
- Team Competencies
Team Values
We did a few workshops where we identified a set of values:
- Leader to Team: How I expect the team to perform
- Team to Leader: How the Team expects me to perform
- Team to Team: How we expect each other to perform
This exercise really helped me understand what was going on within the team and through it I really started to understand some priorities.
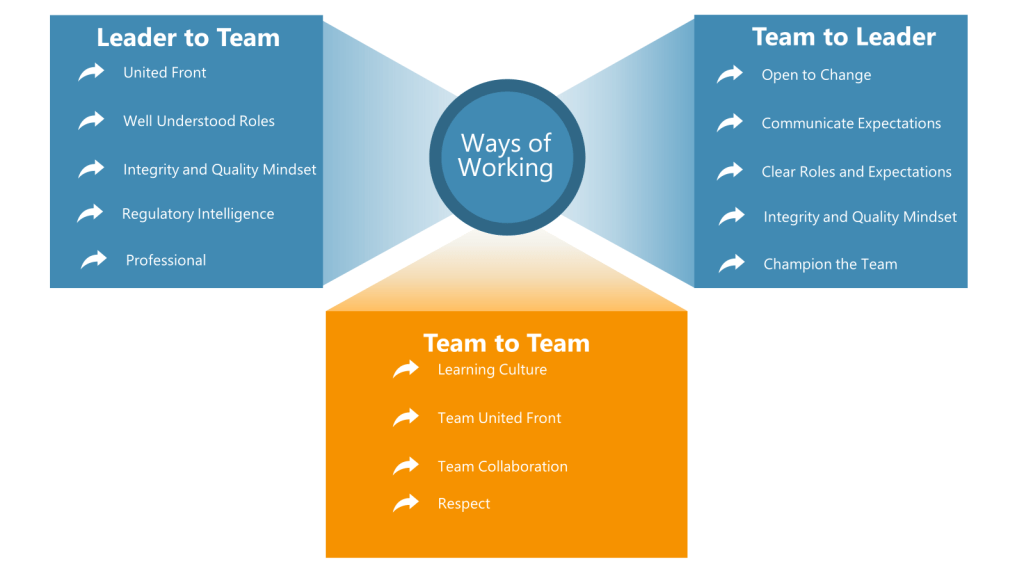
For each of these, we created a Value Statement. Here are some examples.
Value: United Front
Definition: Decisions are made and recorded honestly and transparently. Employees understand decisions and how to execute them. The entire team represents the decisions made, and the decision-making process with one voice.
Desired Behaviors:
- I hold myself accountable for representing the decisions made by the team.
- I work to anticipate and fend off the possibility of failures occurring.
- I engage with decision making and respect the decisions that result.
Value: Open to Change
Definition: Willingness to listen to the team. Actively looking for feedback and input from the team before making decisions that impact the team. Open to changing established ways and revisiting previously made decisions.
Desired Behaviors:
- I will be transparent with decision-making.
- I will create an environment where new ideas are welcome and challenging ideas are encouraged.
- I will include the team in decision-making where applicable.
- I will actively seek out individual and group feedback to enable continuous improvements.
Value: Learning Culture
Definition: Share lessons learned from projects so team can grow together and remain aligned. Engage in knowledge-sharing sessions.
Desired Behaviors:
- I will share lessons learned from each project with the wider QEV team via teams channel &/or weekly team meetings.
- I will encourage team members to openly share their experiences, successes, and challenges without fear of judgement.
- I will update RAID log with decisions made by the team.
- I will identify possible process improvements and update the process improvement tracker
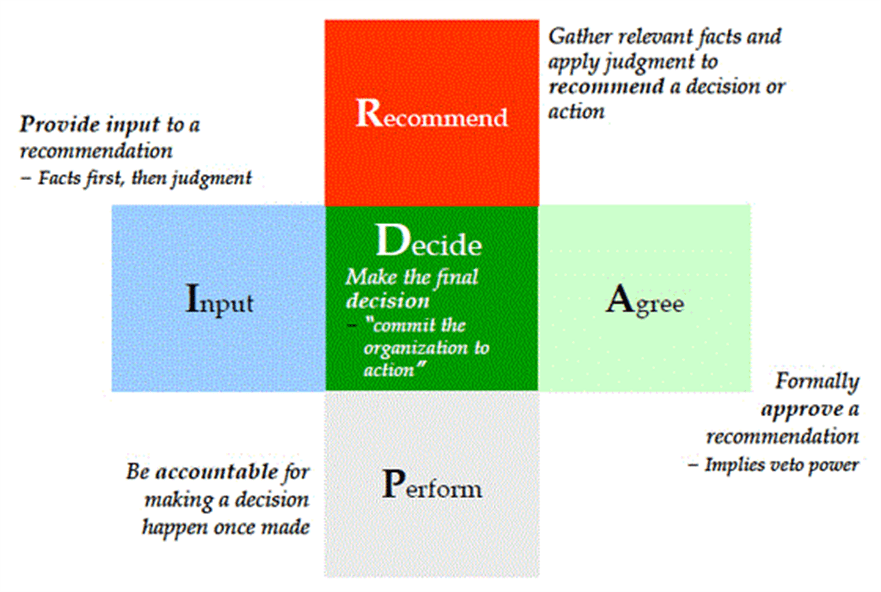
Team Decision Making
Currently working with the team to define decision-making, introducing the RAPID model and working on a matrix of decisions.
Team Competencies
Starting with technical skills we are defining our core competencies. Next, we will tackle, with the larger quality organization, the soft skill side of the equation. This is definitely a work in progress.
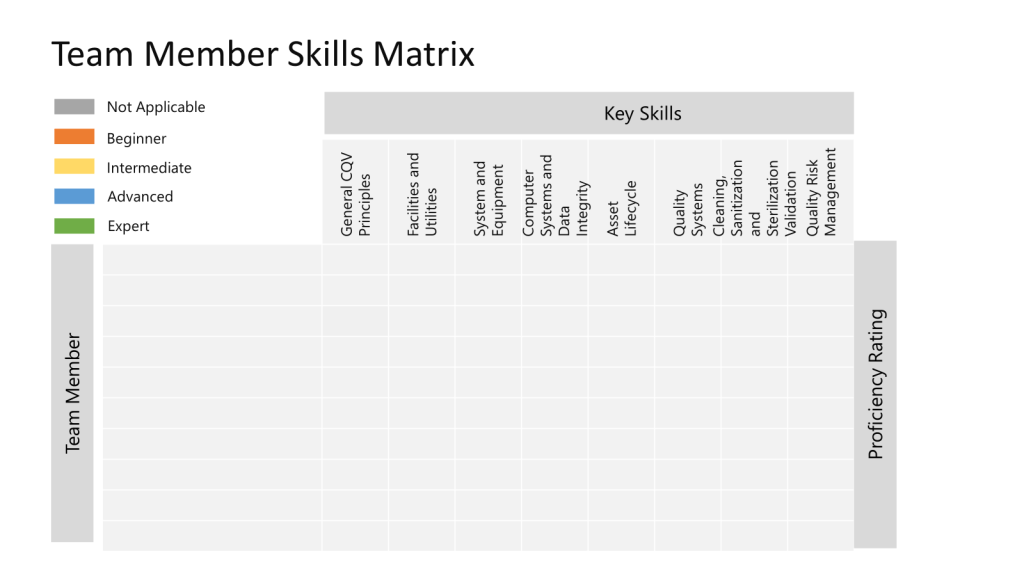
|
Skill Area |
Key Aspects |
Proficiency Levels |
|
||||
|
Beginner |
Intermediate |
Advanced |
Expert |
||||
|
General CQV Principles |
� Modern process validation and guidance � Validation design and how to reduce variability |
� Able to review a basic protocol � Able to review/approve Validation document deliverables. � Understands the importance of a well-defined URS. |
� Able to be QEV lead in a small project � Able to answer questions and guide others in QEV � Participates in process improvement � Able to review and approve RTM/SRs |
� Able to be QEV lead in a large project project � Trains and mentors others in QEV � Leads process improvement initiatives � Able to provide Quality oversight on the creation of Validation Plans for complex systems and/or projects |
� Sets overall CQV strategy � Recognized as an expert outside of JEB |
||
|
Facilities and Utilities |
� Oversee Facilities, HVAC and Controlled Environments � Pharma Water and WFI � Pure Steam, Compressed Air, Medical Gases |
� Understands the principles and GMP requirements |
� Applies the principles, activities, and deliverables that constitute an efficient and acceptable approach to demonstrating facility fitness-for-use/qualification |
� Guide the Design to Qualification Process for new facilities/utilities or the expansion of existing facilities/utilities |
� Able to establish best practices |
||
|
Systems and Equipment |
� Equipment, including Lab equipment |
� Understands the principles and GMP requirements |
� Principles, activities, and deliverables that constitute an efficient and acceptable approach to demonstrating equipment fitness-for-use/qualification |
� Able to provide overall strategy for large projects � Able to be QEV lead on complex systems and equipment. |
� Able to establish best practices |
||
|
Computer Systems and Data Integrity |
� Computer lifecycle, including validation |
� Understands the principles and GMP requirements |
� Able to review CSV documents � Apply GAMP5 risk based approach � Day-to-day quality oversight |
� Able to provide overall strategy for a risk based GAMP5 approach to computer system quality |
� Able to establish best practices |
||
|
Asset Lifecycle |
� Quality oversight and decision making in the lifecycle asset lifecycle: Plan, acquire, use, maintain, and dispose of assets |
� Can use CMMS to look up Calibrations, Cal schedules and PM schedules |
� Quality oversight of asset lifecycle decisions � Able to provide oversight on Cal/PM frequency � Able to assess impact to validated state for corrective WO’s. |
� Able to establish asset lifecycle for new equipment classes � Establish risk-based PM for new asset classes |
� Establish asset lifecycle approach |
||
|
Quality Systems |
� SOP/WI and other GxP Documents � Deviation � Change Control |
� Able to use the eQMS |
� Deviation reviewer (minor/major) � Change Control approver � Document author/approver |
� Deviation reviewer (critical) � Manage umbrella/Parent changes |
� Able to set strategic direction |
||
|
Cleaning, Sanitization and Sterilization Validation |
� Evaluate and execute cleaning practices, limit calculations, scientific rationales, and validation documents � Manage the challenges of multi-product facilities in the establishment of limits, determination of validation strategies, and maintaining the validated state |
� Differentiate the requirements for cleaning and sterilization validation when using manual, semi-automatic, and automatic cleaning technologies � Review protocols |
� Identify and characterize potential residues including product, processing aids, cleaning agents, and adventitious agents � Understand Sterilization principles and requirements � Create, review and approve scientifically sound rationales, validation protocols, and reports |
� Manage and remediate the pitfalls inherent in cleaning after the production of biopharmaceutical and pharmaceutical products |
� Define cleaning/sterilization validation strategy to meet GMP requirements |
||
|
Quality Risk Management |
� Apply QRM principles according to Q9 |
� Participate in a risk assessment |
� Determine appropriate tools � Establish risk-based decision-making tools |
� Set risk-based approaches |
� Define risk management program for CQV activities |
||

7 thoughts on “Where I am at”