November was an exciting month for change management!
ICH Q12 “Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management” was adopted by the ICH in Singapore, which means Q12 is now in Stage 5, Implementation. Implementation should be interesting as concepts like “established conditions” and “product lifecycle management” which sit at the core of Q12 are still open for interpretation as Q12 is implemented in specific regulatory markets.
And then, to end the month, PIC/S published draft 1 of PI 054-1 “Recommendation on How to Evaluate / Demonstrate the Effectiveness of a Pharmaceutical Quality System in relation to Risk-based Change Management.”
This draft guidance is now in a review period by regulatory agencies. Which means no public comments, but it will be applied on a 6-month trial basis by PIC/S participating authorities, which include the US Food and Drug Administration and other regulators across Europe, Australia, Canada, South Africa, Turkey, Iran, Argentina and more.
This document is aligned to ICH Q10, and there should be few surprised in this. Given PIC/S concern that “ongoing continual improvement has probably not been realised to a meaningful extent. The PIC/S QRM Expert Circle, being well-placed to focus on the QRM concepts of the GMPs and of ICH Q10, is seeking to train GMP inspectors on what a good risk-based change management system can look like within the PQS, and how to assess the level of effectiveness of the PQS in this area” it is a good idea to start aligning to be ahead of the curve.
“Changes typically have an impact assessment performed within the change control system. However, an impact assessment is often not as comprehensive as a risk assessment for the proposed change.”
This is a critical thing that agencies have been discussing for years. There are a few key takeaways.
- The difference between impact and risk is critical. Impact is best thought of as “What do I need to do to make the change.” Risk is “What could go wrong in making this change?” Impact focuses on assessing the impact of the proposed change on various things such as on current documentation, equipment cleaning processes, equipment qualification, process validation, training, etc. While these things are very important to assess, asking the question about what might go wrong is also important as it is an opportunity for companies to try to prevent problems that might be associated with the proposed change after its implementation.
- This 8 page document is really focusing on the absence of clear links between risk assessments, proposed control strategies and the design of validation protocols.
- The guidance is very concerned about appropriately classifying changes and using product data to drive decisions. While not specifying it in so many words, one of the first things that popped to my mind was around how we designate changes as like-for-like in the absence of supporting data. Changes that are assigned a like-for-like classification are often not risk-assessed, and are awarded limited oversight from a GMP perspective. These can sometimes result in major problems for companies, and one that I think people are way to quick to rush to.
Much of my thoughts on implementing this can be found in my presentation on change management and change control.
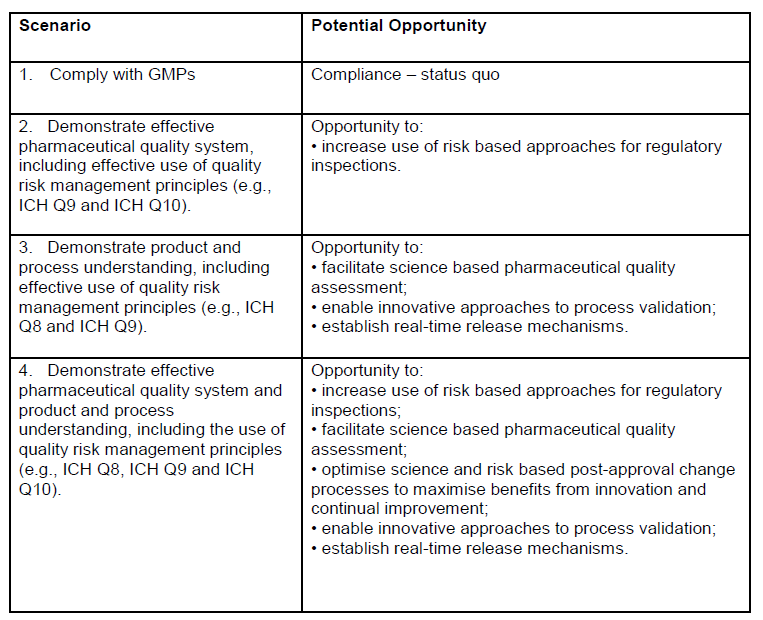
It is fascinating to look at appendix 1, which really lays out some critical goals of this draft guidance: better risk management, real time release, and innovative approaches to process validation. This is sort of the journey we are all on.