Both Curious Cat and A Lean Journey tell me that the ASQ Influential Voices blogs are covering change management. I do love a good blog carnival, and change management is sort of my thing, so I am going to jump in.
It’s often said that people don’t resist “change” so much as they resist “being changed.” So, the job of change management is clear: In a nutshell, you must explain why the affected people should want to change, and thereby cultivate readiness instead of resistance.
What are some recommended strategies or tactics to help achieve successful change management?
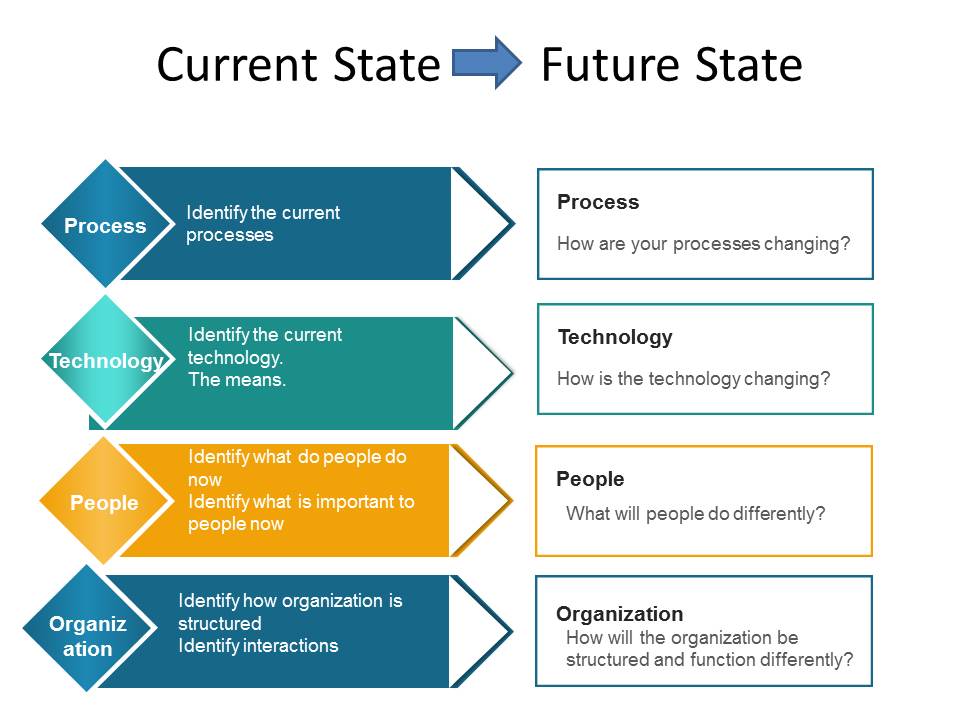
My first piece, of advice, abandon the idea that change management only involves people. Just as all systems are made of people, organization, process and technology; all changes impact all four and need to be viewed holistically.
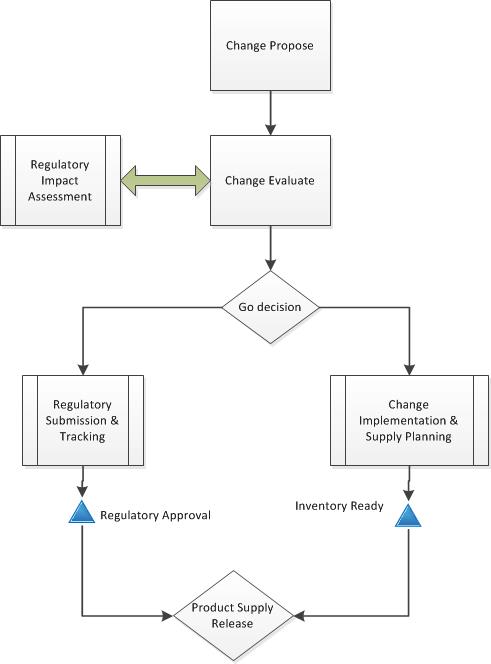
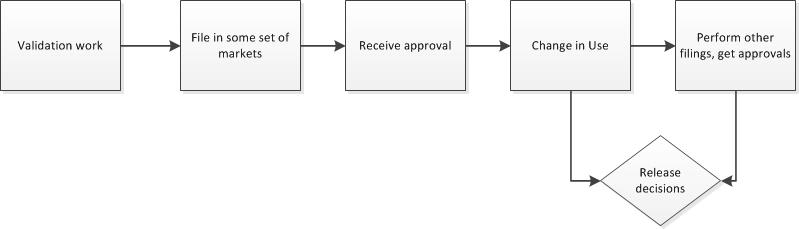
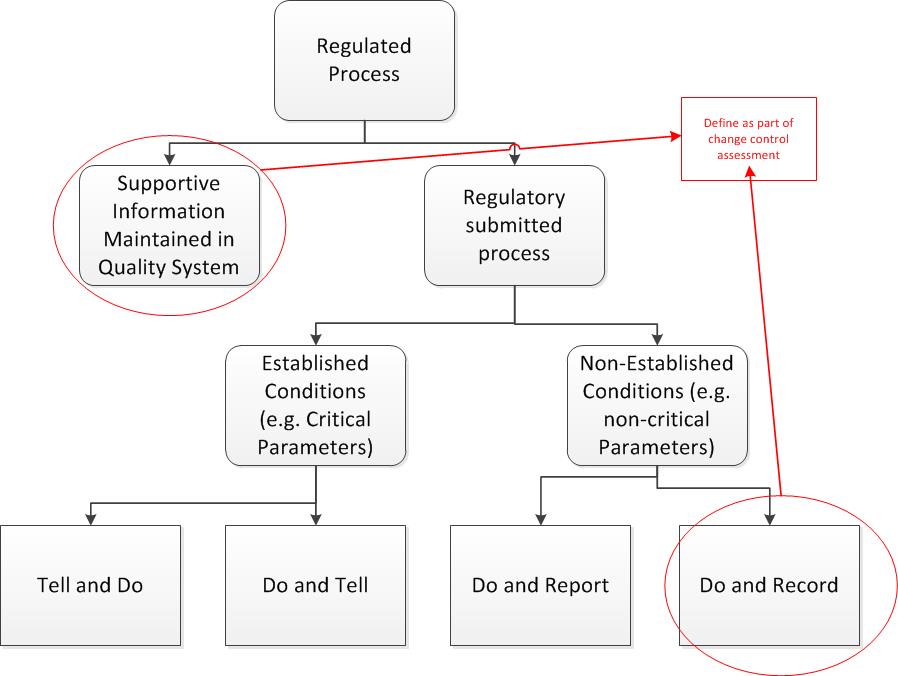
Second, get rid of the artificial barriers between change management and change control. Change management is the how of change – assess, handle and release. Change control is the what, the execution steps. Remember that all changes are really just projects, and vice versa. The level of change determines the level of activity.
| Level of Change | Change Management | Change Control |
| Transactional | Minor | Few
Closely clustered |
| Operational | Major | Several
Across several areas |
| Transformational | Fundamental | Many
Iterative Often in waves |
Simplify your variety of change controls and strive for scalability within one change management (and control) system. Utilize the levers, which include: regulatory (compliance), product release and risk.
Knowledge Management
Change Management and Knowledge Management are closely entwined. An effective change management system includes active knowledge management, in which information from multiple sources is integrated to identify stimuli for changes needed to improve product and/or process robustness.
There are key interactions with document management and training.
Risk Management
Risk management enables changes and helps assess:
- The proposed change
- The effectiveness of the change once implemented

Propose the Change
Make it SMART:
- Specific – The proposal needs to be accurate and leave no doubt as to what the change will achieve.
- Measurable – How will the system owner (sponsor) know when the project is complete.
- Achievable – Make the change as small as possible after all it is easier to eat an elephant one bite at a time. It is far easier to manage a few smaller change than one big one. This is why operational and transformational changes are many changes and often iterative.
- Realistic – Make the change easy to deliver, if it is over complicated then it is likely to hit problems and run over budget, be delivered late or of poor quality.
- Timely – Does the change have to be complete by a certain date? If so put it in the scope that the project has to be complete by that date. Are there dependencies and independencies?
Evaluate
The change Project team leverages SMEs to harness the collective intelligence (synergy) for the benefit of the site.
- Relevancy – The information gathered is of value
- Reliability – The process by which the information is collected should be consistent
- Accuracy – The data should be expressed in a manner that most accurately reflects its information content
- Efficiency – The design and implementation of the tasks should minimize the burden
Evaluates all four areas (process, technology, people and organization). Includes communication of the change and training.
| Vision | Importance |
| What is the vision for this change | Why is this change important to our organization |
| Success Measurements | Process Measurements |
| How will we measure success | How will we show progress towards our vision? |
| Who and what is affected? | |
| What people, departments and processes need to change in order to realize our vision? | |
| How will we support people? | |
| What actions will we do to support people through the change? | |
| What is our plan? | |
| Detailed action plan | |
Build in effectiveness reviews to your plan.
Implement
Execute the change plan, provide evidence of completion. Escalate significant risks or delays.
Close
Ensure change plan was executed and benefits realized.
Hold a lessons learned.

Conclusion
Change management is a system. It should have its own cycles of improvement and grow as you execute changes. Change is a fundamental pillar of a quality system and spending the time to build a robust system will reap dividends and prove itself a good idea again and again.