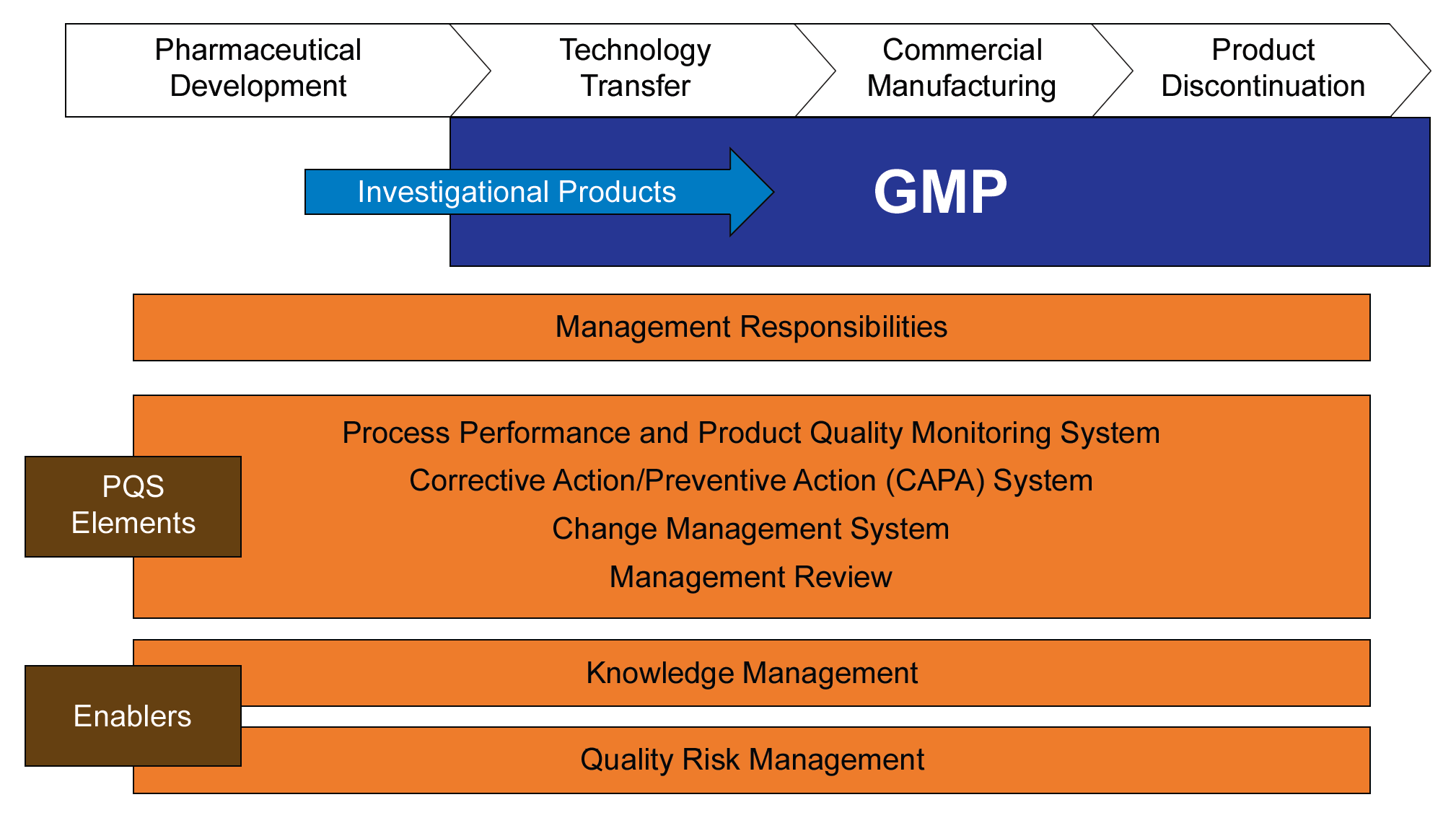
Yesterday, the FDA finalized the ICH Q7 Q&A Guidance on GMPs following its endorsement by the regulatory agencies participating in the ICH in June 2015.
Two-and-a-half-years. And I sometimes wonder why the ICHs aren’t more broadly adopted or why some of my colleagues are a little pessimistic about their impact on this industry
However, re-reading these questions and answers gave me a good topic.

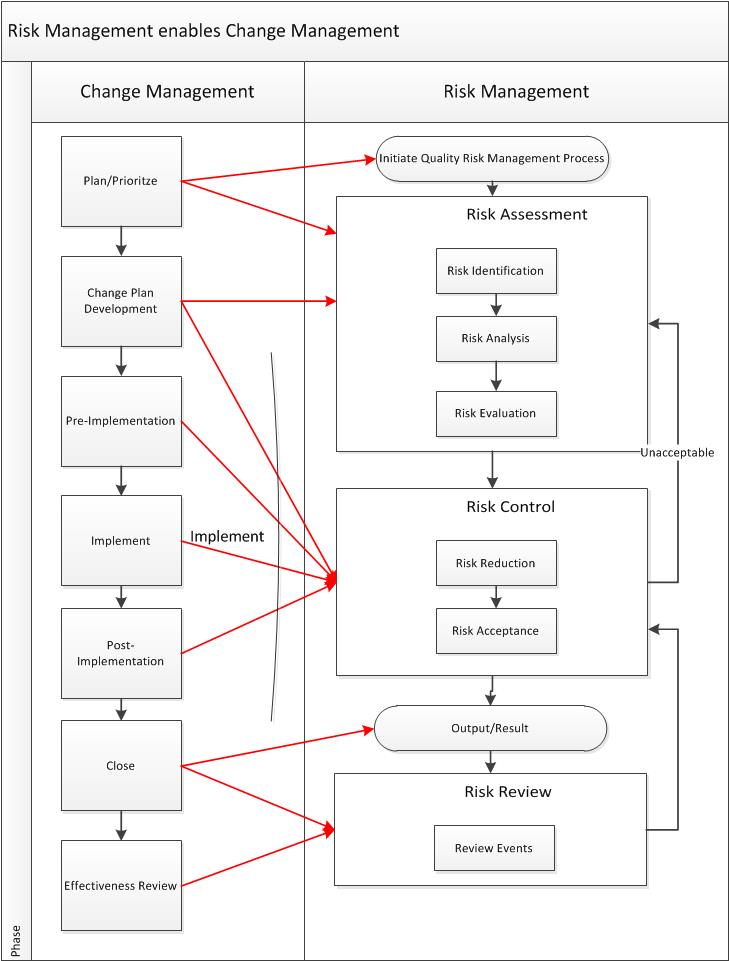
With complex and virtual supply chains, with world wide distribution, it is important to understand who needs to be communicated about what changes.
This communication should be evaluated for it’s directionality. It helps to break down your types of changes and determine what are:
- Consult – those changes where the other site needs to provide an assessment. For example, if a change impacts testing that is conducted at another site, or it impacts the way the next site will receive the material (don’t forget ERP changes). These communications are always push.
- Inform – the other site needs to be aware but will not need to take any action. A great example of this notifying the QP.
Include your suppliers in this process as well, and ensure your suppliers are also appropriately communicating. Include this in your quality/technical agreements or contract terms.