In February I will be presenting at the 2020 ASQ Lean and Six Sigma Conference in Phoenix on Sustaining Change – Executing a Sustainability Plan.
Here’s the presentation summary:
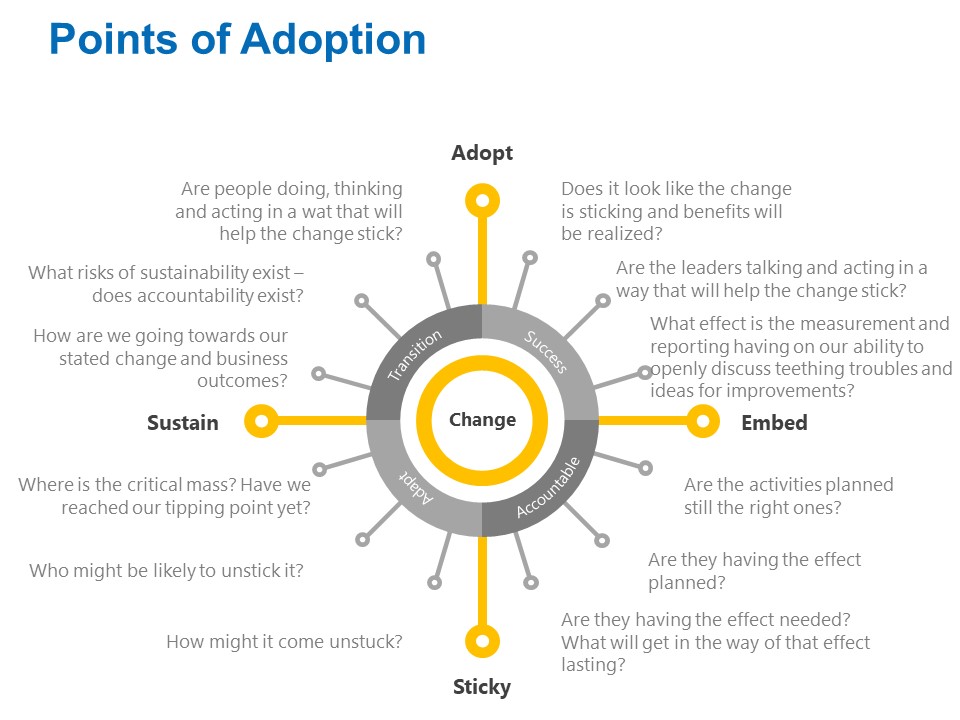
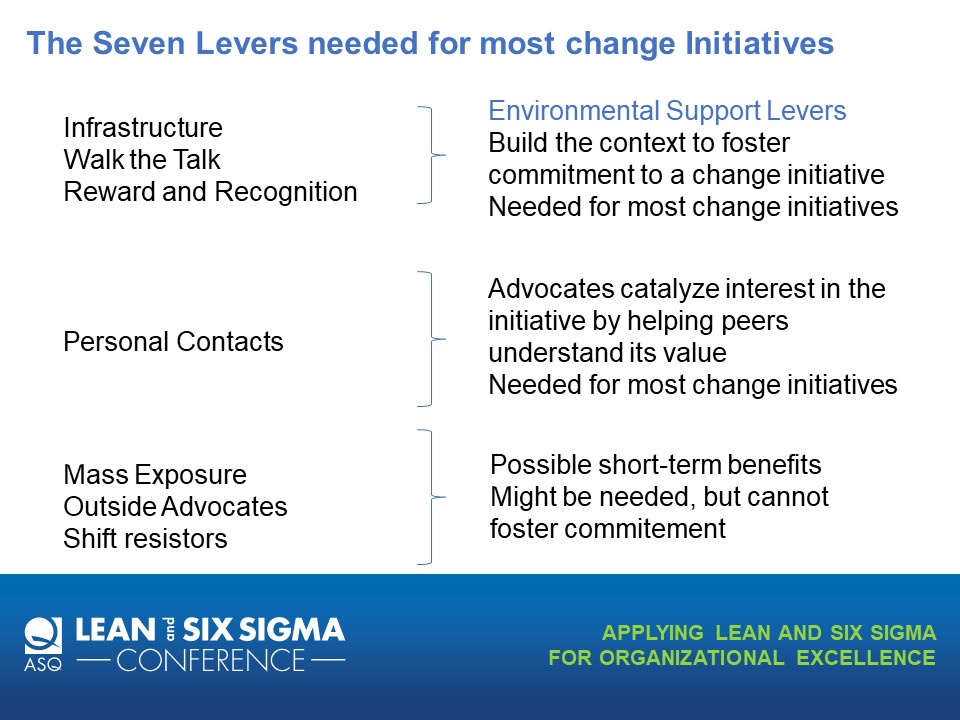
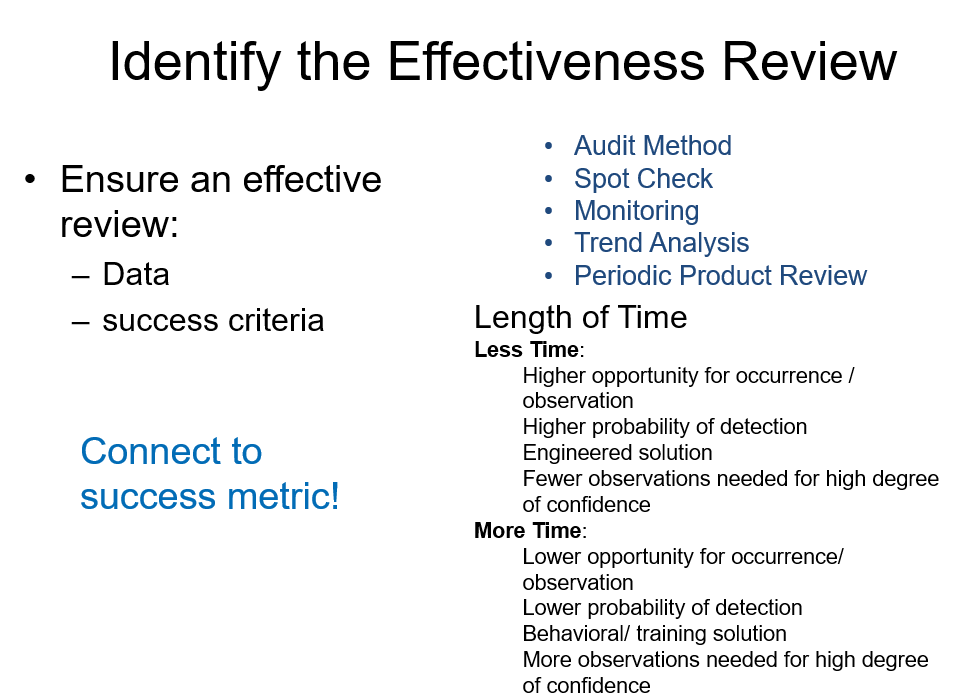
For Lean and Six Sigma projects a central question should always be “how do we sustain this change?” Sustainability is a major part of all the major change models but is often the easiest to neglect. This session will engage the participant in building a Sustainability Plan, a key tool to ensure the change is anchored and embedded in the organization. Through three case study examples of changes at the three major change levels -transactional, organizational and transformational – the participant will gain the knowledge to create and execute an effective change plan.
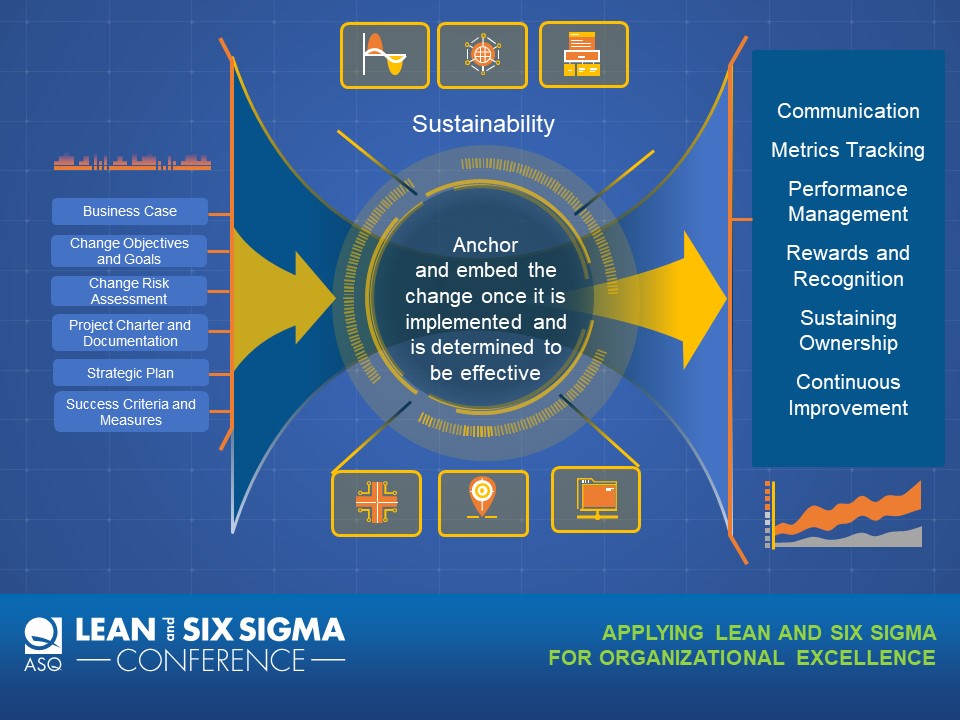
During this session examples will be given for each component of a sustainability plan:
- Communication: Mechanisms for persuasive communication and ongoing socialization of the change, rites of parting (saying goodbye to the old ways of doing things), and rites of enhancement (acknowledgment of quick wins and continued adoption)
- Metrics Tracking: How to identify and execute consistent and effective ongoing measurement and results reporting to track progress and ensure sustained results • Performance Management: Process for observing and objectively measuring desired behaviors and attitudes, including performance appraisal process, promoting, demoting and transferring, and training and development
- Rewards and Recognition: Program of intrinsic and extrinsic incentives to reinforce desired behaviors and attitudes
- Sustaining Ownership: Consistent process for ensuring sustained ownership of the change through the ongoing transfer of experience and knowledge
- Continuous Improvement: Mechanisms for responding to changing requirements and implementing improvements based on feedback, observations, and metrics
The following questions will be explored, and tools for finding answers will be provided:
- How should organizational achievements reinforcing the change be commemorated
- What behaviors should be observed and measured on a regular basis?
- What results should be observed and measured on a regular basis?
- What metrics should be used for measuring behaviors and results?
- What mechanisms should be used for reporting results? • What criteria should be used to allocate rewards and promotion?
- What mechanisms should be used for training, coaching, and role modeling?
- What processes and procedures should be put in place to ensure sustained ownership of the change?
- What continuous improvement mechanisms will address low adoption rates and ensure the change becomes part of the organization’s normal functioning?
At the end of the session the user will have a template for creating a sustainability plan and will have been provided tools to successfully execute the sustainability phase of a change.
Learning Objectives 1. Assess the role of sustainability in the major change management methodologies and apply to lean and six sigma projects. 2. Facilitate the sustainability phase of change management. 3. Compose a sustainability plan.