On Wednesday the United States set a devastating new record in the coronavirus pandemic: 3,124 people dead in one day. This was the first time the daily number of deaths has exceeded 3,000 but I fear it will not be the last. There are over 260k deaths in the US so far, over 1.5 million deaths worldwide. This is crippling, and it is difficult to go day-by-day with the pain of this suffering.
And yet, we need to work, support our families and communities. Get the job done. Amidst all that it is important to remember that is important to grieve and it is okay to be angry.
People grieve in diverse ways with different emotions, from anger, to depression to hopelessness, to resentment over what has been taken from them. Combined with the isolation of the pandemic, this is a recipe for poor mental health and poor coping mechanisms. And then there is a question of just how much and what sort of coping is good. Two-hundred-and-sixty thousand people are dead and there is a lot of evidence this is an underreport and a lot more people are going to die.
I hope you understand that I am angry. All day long. And it is a struggle not to bring that anger to work, not to let it twist my relationships. Yet that anger always exists.
I linked earlier this week to an article on mental health. It is particularly important to make this part of our organizations. Burnout must have a systematic fix.
What we need to give permission to, give space to, is a recognition that we are not in an okay state. And it may not be okay for a very long while, long after vaccines are widely available, and we return to the office.
It is okay to have taken a step back from obligations. I have not, for example, been writing much on this blog. It just did not work for me. Be kind to yourself and be okay with the things you must do less of. And when you are ready, go back to it.
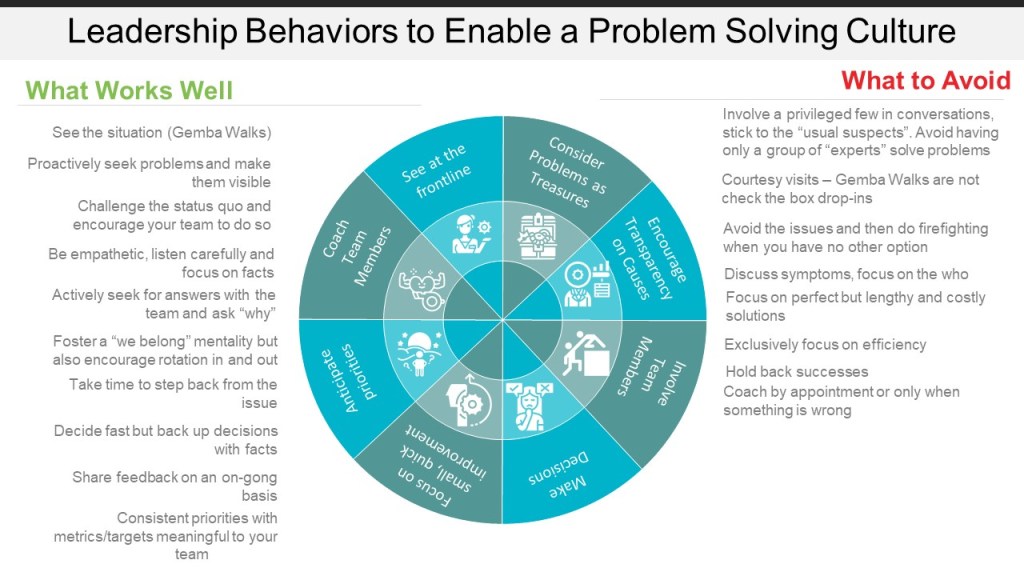
Anger and Culture
Our organizational cultures are full of anger. What we must do is work to establish mechanisms to assure that anger is directed at issues or situations, not people. This will build psychological safety, enable good decisions and enhance our problem solving culture.
Some things we should do:
- Acknowledge what is happening: Senior leadership needs to be working from compassion and generosity and taking real steps to address.
- Treat toxic positivity as a bias: Toxic positivity is the assumption, either by one’s self or others, that despite a person’s emotional pain or difficult situation, they should only have a positive mindset. This is especially important as we have talent discussions, evaluate performance, and perform other managerial tasks.
- Have systems around burnout
- Focus on decision making quality
- Build employee judgement feedback loops
We are not done. This winter will be very hard for many. As leaders we need to be ensuring our organizations can get through this and then leverage what we’ve learned to build a better culture.