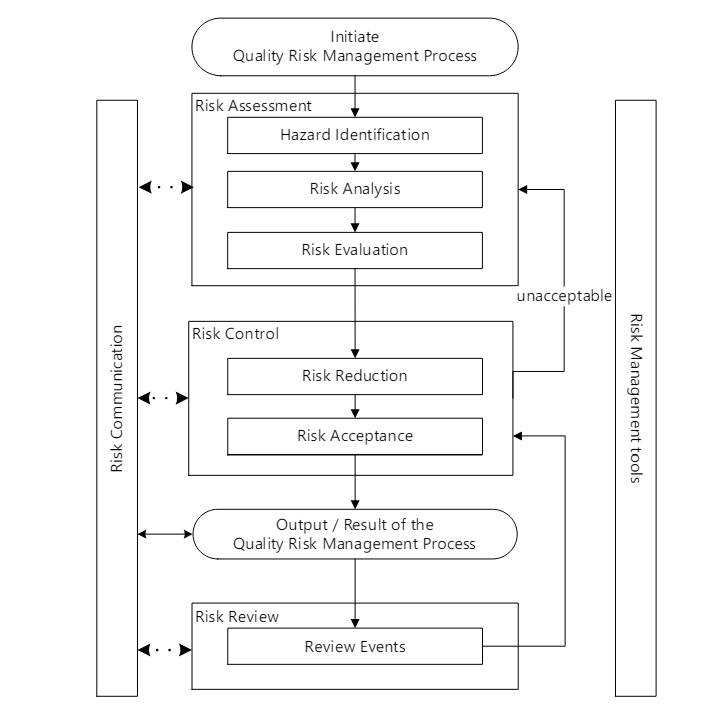
The ICH Q9 guideline on Quality Risk Management (QRM), including its revised version ICH Q9(R1), addresses the concept of uncertainty as a critical component in risk management within the pharmaceutical industry.
Understanding Uncertainty in ICH Q9
Uncertainty in the context of ICH Q9 refers to the lack of complete knowledge about a process and its expected or unexpected variability. This uncertainty can stem from various sources, including gaps in knowledge about pharmaceutical science, process understanding, and potential failure modes.
Key Points on Uncertainty from ICH Q9(R1)
Sources of Uncertainty:
- Knowledge Gaps: Incomplete understanding of the scientific and technical aspects of processes.
- Process Variability: Both expected and unexpected changes in process performance.
- Failure Modes: Unidentified or poorly understood potential points of failure in processes or systems.
Managing Uncertainty:
- Risk-Based Decision Making: The guideline emphasizes that decisions should be made based on the level of uncertainty, importance, and complexity of the situation. This means that more formal and structured approaches should be used when uncertainty is high.
- Formality in QRM: ICH Q9(R1) introduces the concept of formality as a spectrum, suggesting that the degree of formality in risk management activities should be commensurate with the level of uncertainty. Less formal methods may be appropriate for well-understood processes, while highly structured methods are necessary for areas with high uncertainty.
Reducing Subjectivity:
- The guideline acknowledges that subjectivity can impact the effectiveness of risk management. It recommends strategies to minimize subjectivity, such as using well-recognized risk assessment tools and involving cross-functional teams to provide diverse perspectives.
Continuous Improvement:
- ICH Q9(R1) stresses the importance of continual improvement in risk management processes. This involves regularly updating risk assessments and control measures as new information becomes available, thereby reducing uncertainty over time.
Practical Implementation
In practice, managing uncertainty within the framework of ICH Q9 involves:
- Conducting thorough risk assessments to identify potential hazards and their associated risks.
- Applying appropriate risk control measures based on the level of uncertainty and the criticality of the process.
- Documenting and reviewing risk management activities to ensure they remain relevant and effective as new information is obtained.
Conclusion
The ICH Q9 approach to uncertainty underscores the importance of a structured, knowledge-based approach to risk management in the pharmaceutical industry. By addressing uncertainty through rigorous risk assessments and appropriate control measures, organizations can enhance the reliability and safety of their processes and products, ultimately safeguarding patient health and safety.