This past week the FDA issued a draft guidance “Post Approval Changes to Drug Substances” from the Center for Drug Evaluation and Research on post-approval changes for drug substances to provide clarity to holders of drug master files and holders of new and generic drug applications on which reporting category manufacturing changes fall into as well as the information required to support these changes.
Like any guidance this is supposed to “describe the Agency’s current thinking on a topic” and in this case this is a pretty important guidance as it is the first on the subject of change management published after the draft of Q12. It also clearly builds on the concepts of Q11 and is the first time the agency has published a guidance on drug substance post approval changes.
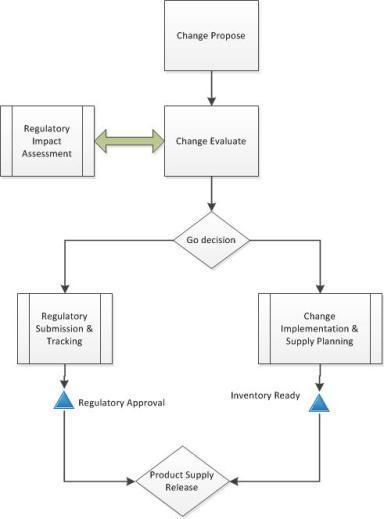
The guidance covers changes to facility; scale and equipment changes; specification changes to starting materials; synthetic manufacturing process changes; and, changes to the container closure systems for the drug substance.
It uses the traditional breakdown of major changes (prior approval supplement category), Moderate changes (changes-being-effected-30 (CBE-30) category) and Minor changes (annual report).
The guidance provides some solid requirements for risk assessments, and requires the risk assessment be included with the filing of change. This will require some companies to improve their risk management process, and may cause some to question decision making about their internal formulas for level of effort and the formality of risk management.
The deadline for public comment on the draft is Nov. 13. Submit comments to the docket at: https://www.regulations.gov/docket?D=FDA-2018-D-3152.