Through the skilled work of a very helpful FOIA officer at the FDA I have been reviewing the 2020 483 and EIR for the pre-approval inspection at the Sanofi Framingham, MA site that recently received a Warning Letter:
The 2020 pre-approval inspection (PAI) of Sanofi’s facility in Framingham, MA, uncovered critical deviations that exposed systemic weaknesses in contamination controls, equipment maintenance, and quality oversight. These deficiencies, documented in FDA Form 483 (FEI 1220423), violated 21 CFR 211 regulations and FDA Compliance Program 7346.832 requirements for PAIs. The facility’s failure to address these issues and to make systeatic changes over time (and perhaps backslide, but that is conjecture) contributed to subsequent regulatory actions, including a 2022 Form 483 and the 2024 FDA warning letter citing persistent CGMP violations. This analysis traces the 2020 findings to their regulatory origins, examines their operational consequences, and identifies lessons for PAI preparedness in high-risk API manufacturing.
Regulatory Foundations of Pre-Approval Inspections
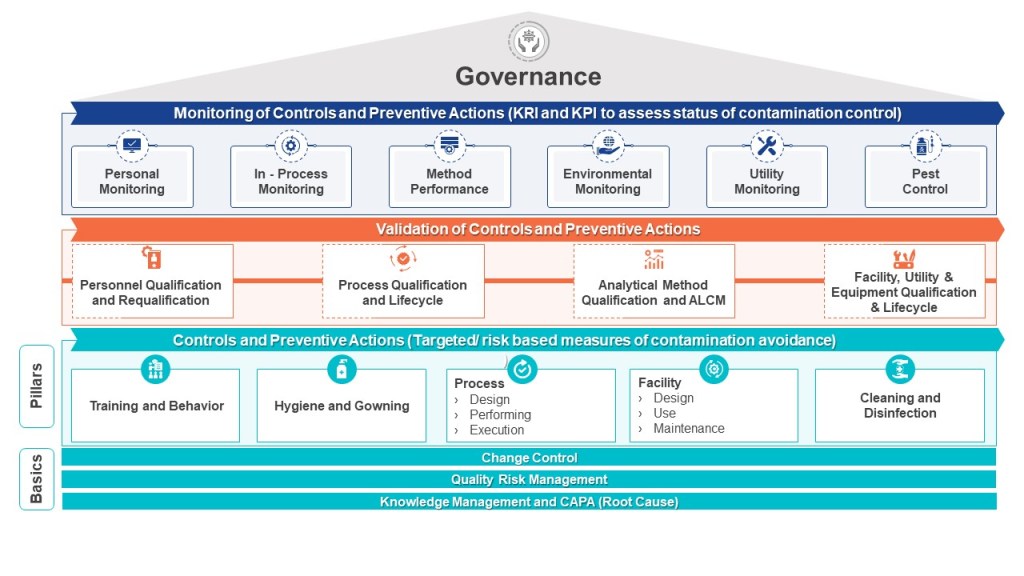
The FDA’s PAI program operates under Compliance Program 7346.832, which mandates rigorous evaluation of facilities named in NDAs, ANDAs, or BLAs. Three pillars govern these inspections:
- Commercial Manufacturing Readiness: PAIs assess whether facilities can reliably execute commercial-scale processes while maintaining CGMP compliance. This includes verification of validated equipment cleaning procedures, environmental monitoring systems, and preventive maintenance programs. The FDA prioritizes sites handling novel APIs, narrow therapeutic index drugs, or first-time applications—criteria met by Sanofi’s production of drug substances.
- Application Conformance: Inspectors cross-validate submission data against actual operations, focusing on batch records, process parameters, and analytical methods. Discrepancies between filed documentation and observed practices constitute major compliance risks, particularly for facilities like Sanofi that utilize complex biologics manufacturing processes.
- Data Integrity Assurance
Per 21 CFR 211.194, PAIs include forensic reviews of raw data, equipment logs, and stability studies. The 2020 inspection identified multiple QC laboratory lapses at Sanofi that undermined data reliability—a red flag under FDA’s heightened focus on data governance in PAIs.
Facility Maintenance Deficiencies
Sterilization Equipment Contamination
On September 2, 2020, FDA investigators documented (b)(4) residue on FB-2880-001 sterilization equipment and its transport cart—critical infrastructure for bioreactor probe sterilization. The absence of cleaning procedures or routine inspections violated 21 CFR 211.67(a), which mandates written equipment maintenance protocols. This lapse created cross-contamination risks for (b)(4) drug substances, directly contradicting the application’s sterility claims.
The unvalidated cleaning process for those chambers further breached 21 CFR 211.63, requiring equipment design that prevents adulteration. Historical data from 2008–2009 FDA inspections revealed similar sterilization issues at Allston facility, suggesting systemic quality control failures which suggests that these issues never were really dealt with systematically across all sites under the consent decree.
Environmental Control Breakdowns
The August 26, 2020 finding of unsecured pre-filters in Downflow Booth —a critical area for raw material weighing—exposed multiple CGMP violations:
- 21 CFR 211.46(b): Failure to maintain HEPA filter integrity in controlled environments
- FDA Aseptic Processing Guidance: Loose filters compromise ISO 5 unidirectional airflow
- 21 CFR 211.42(c): Inadequate facility design for preventing material contamination
Ceiling diffuser screens in Suite CNC space with unsecured fasteners exacerbated particulate contamination risks. The cumulative effect violated PAI Objective 1 by demonstrating poor facility control—a key factor in the 2024 warning letter’s citation of “unsuitable equipment for microbiologically controlled environments”.
Quality Control Laboratory Failures
Analytical Balance Non-Compliance
The QC microbiology laboratory’s use of an unqualified balance breached multiple standards:
- 21 CFR 211.68(a): Lack of calibration for automated equipment
- USP <41> Guidelines: Failure to establish minimum weigh limits
- FDA Data Integrity Guidance (2018): Unguaranteed accuracy of microbiological test results
This deficiency directly impacted the reliability of bioburden testing data submitted in the application, contravening PAI Objective 3’s data authenticity requirements.
Delayed Logbook Reviews
Three QC logbooks exceeded the review window specified in the site’s procedure:
- Temperature logs for water baths
- Dry state storage checklists
The delays violated 21 CFR 211.188(b)(11), which requires contemporaneous review of batch records. More critically, they reflected inadequate quality unit oversight—a recurring theme in Sanofi’s 2024 warning letter citing “lackluster quality control”.
And if they found 3 logbooks, chances are there were many more in an equal state.
Leak Investigations – A Leading Indicator
there are two pages in the EIR around leak deviation investigations, including the infamous bags, and in hindsight, I think this is an incredibly important inflection point from improvement that was missed.
The inspector took the time to evaluate quite a few deviations and overall control strategy for leaks and gave Sanofi a clean-bill of health. So we have to wonder if there was not enough problems to go deep enough to see a trend or if a sense of complacency allowed Sanofi to lower their guard around this critical aspect of single use, functionally closed systems.
2022 Follow-Up Inspection: Escalating Compliance Failures
The FDA’s July 2022 reinspection of Sanofi’s Framingham facility revealed persistent deficiencies despite corrective actions taken after the 2020 PAI. The inspection, conducted under Compliance Program 7356.002M, identified critical gaps in data governance and facility maintenance, resulting in a 2-item Form FDA 483 and an Official Action Indicated (OAI) classification – a significant escalation from the 2020 Voluntary Action Indicated (VAI) status.
Computerized System Control Failures
The FDA identified systemic weaknesses in data integrity controls for testers used to validate filter integrity during drug substance manufacturing. These testers generated electronic logs documenting failed and canceled tests that were never reviewed or documented in manufacturing records. For example:
- On June 9, 2022, a filter underwent three consecutive tests for clarification operations: two failures and one cancellation due to operator error (audible “hissing” during testing). Only the final passing result was recorded in logbooks.
- Between 2020–2022, operators canceled 14% of tests across testers without documented justification, violating 21 CFR 211.68(b) requirements for automated equipment review.
The firm had improperly classified these testers as “legacy electronic equipment,” bypassing mandatory audit trail reviews under their site procedure. I am not even sure what legacy electronic equipment means, but this failure contravened FDA’s Data Integrity Guidance (2018), which requires full traceability of GxP decisions.
Facility Degradation Risks
Multiple infrastructure deficiencies demonstrated declining maintenance standards:
Grade-A Area Compromises
- Biological Safety Cabinet: Rust particles and brown residue contaminated interior surfaces used for drug substance handling in April 20223. The material was later identified as iron oxide from deteriorating cabinet components.
- HVAC System Leaks: A pH probe in the water system leaked into grade-D areas, with standing water observed near active bioreactors3.
Structural Integrity Issues
- Chipped epoxy floors in grade-C rooms created particulate generation risks during cell culture operations.
- Improperly sloped flooring allowed pooling of rinse water adjacent to purification equipment.
These conditions violated 21 CFR 211.42(c), requiring facilities to prevent contamination through proper design, and demonstrated backsliding from 2020 corrective actions targeting environmental controls.
Regulatory Reckoning
These cultural failures crystallized in FDA’s 2024 citation of “systemic indifference to quality stewardship”. While some technological upgrades provided tactical fixes, the delayed recognition of cultural rot as root cause transformed manageable equipment issues into existential compliance threats—a cautionary tale for pharmaceutical manufacturers navigating dual challenges of technological modernization and workforce transition.
Conclusion: A Compliance Crisis Decade
The Sanofi case (2020–2024) exemplifies the consequences of treating PAIs as checklist exercises rather than opportunities for quality system maturation. The facility’s progression from 483 observations to OAI status and finally warning letter underscores three critical lessons:
- Proactive Data Governance: Holitisic data overnance and data integrity, including audit trail reviews that encompass all GxP systems – legacy or modern.
- Infrastructure Investment: Episodic maintenance cannot replace lifecycle-based asset management programs.
- Cultural Transformation: Quality metrics must drive executive incentives to prevent recurrent failures.
Manufacturers must adopt holistic systems integrating advanced analytics, robust knowledge management, and cultural accountability to avoid a costly regulatory debacle.
PAI Readiness Best Practices
Pre-Inspection Preparation
- Gap Analysis Against CPGM 7346.832
Facilities should conduct mock inspections evaluating:- Conformance between batch records and application data
- Completeness of method validation protocols
- Environmental monitoring trend reports
- Data Integrity Audits
Forensic reviews of electronic records (e.g., HPLC chromatograms, equipment logs) using FDA’s “ALCOA+” criteria—ensuring data is Attributable, Legible, Contemporaneous, Original, and Accurate. - Facility Hardening
Preventive maintenance programs for critical utilities:- Steam-in-place systems
- HVAC airflow balances
- Water for injection loops
Post-Approval Vigilance
The Sanofi case underscores the need for ongoing compliance monitoring post-PAI:
- Quality Metrics Tracking: FDA-required metrics like lot rejection rates and CAPA effectiveness
- Regulatory Intelligence: Monitoring emerging focus areas through FDA warning letters and guidance updates
- Process Robustness Studies: Continued process verification per 21 CFR 211.110(a)