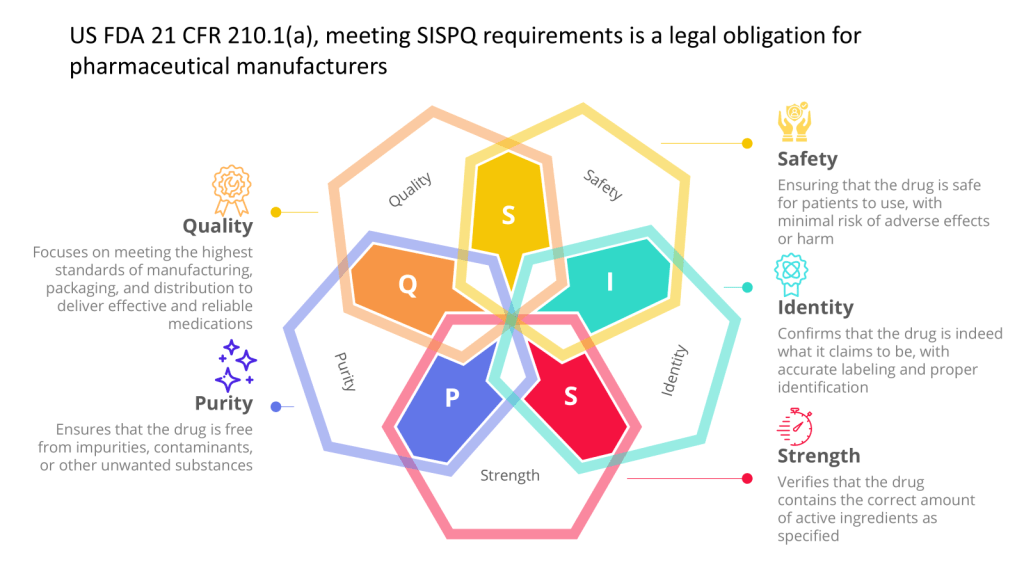
I spend a lot of time discussing uncertainty and how to address it in our quality system and within our organization. However, we often find ourselves at a crossroads, faced with uncertainty and the unknown in our careers – certainly, the last few years have been hard in biotech. My current approach has been to reframe this uncertainty not as an obstacle but as a feature of my journey—something it might have taken me 54 years to learn. I am striving to embrace the concept of “trusting the process” personally and as a quality practitioner so I can navigate life’s twists and turns with greater ease and purpose. As we go into the New Year, here are my current approaches.
The Power of Small Steps
If you are like me, it is easy to get lost in the day-to-day pressures of work. There is always a new issue, a new course correction. It is easy to focus on the overwhelming big picture to our next best steps and forget that the journey counts. My QA problem-solving self often wants to focus on problem-solving and forgets that we must strike a balance between action and acceptance, recognizing that while we can’t control every outcome, we can control our response to each situation. I am working to maintain agency in the present moment while surrendering to the unfolding path ahead.
Embracing Uncertainty as a Catalyst for Growth
Uncertainty, often viewed as a source of anxiety, can actually be a powerful catalyst for growth and innovation. By reframing uncertainty as a feature, we can open ourselves up to new possibilities and unexpected opportunities. This mindset shift encourages us to:
- Remain curious and open-minded
- Adapt more readily to changing circumstances
- Cultivate resilience in the face of challenges
The Art of Experimentation
One practical way to embrace uncertainty is through the practice of running small experiments. These controlled tests allow us to:
- Gather quick feedback
- Minimize risk
- Foster creativity and innovation
We create a culture of continuous learning and improvement by incorporating regular experimentation into our personal and professional lives. This approach is particularly valuable when balancing the demands of serving an organization while pursuing personal growth.
Balancing Service and Growth
The challenge of running small experiments while fulfilling organizational responsibilities is common. Here are some strategies to help strike that balance:
- Integrate experiments into daily work: Look for opportunities to test new ideas or approaches within your existing projects and responsibilities.
- Time-box your experiments: Set aside specific, limited time periods for experimentation to ensure it doesn’t interfere with core duties.
- Communicate with stakeholders: Share your experimental approach, highlighting how it can benefit.
- Learn from successes and failures: Treat every experiment as a learning opportunity, regardless of the outcome.
- Start small and scale up: Begin with low-risk, high-potential experiments and gradually expand based on results and buy-in.
Cultivating Trust in the Process
Trusting the journey is not about blind faith or passivity. Instead, it’s about developing a deep relationship with your wisdom and decision-making process. This trust is built over time through:
- Consistent self-reflection
- Recognizing patterns in your choices and their outcomes
- Staying connected to your core values and goals
- Celebrating small wins and learning from setbacks
As you cultivate this trust, you’ll find yourself better equipped to navigate uncertainty confidently and gracefully.
Embracing the Journey
Trusting the journey can feel counterintuitive in a world that often demands certainty and immediate results. However, by embracing uncertainty as a feature of our growth process, we open ourselves to a richer, more fulfilling experience. Through small experiments, mindful action, and a willingness to surrender to the unknown, we can create a life and career that is both purposeful and adaptable.
Remember, the journey itself is where true growth and discovery happen. By trusting the process and focusing on our next best steps, we can navigate the complexities of life with greater ease and authenticity. So, take that first step, run that small experiment, and trust that the journey will unfold in ways you may never have imagined.
This is my New Year’s plan: to continue to apply to my personal space the skills and mindsets that have made my career so fruitful.