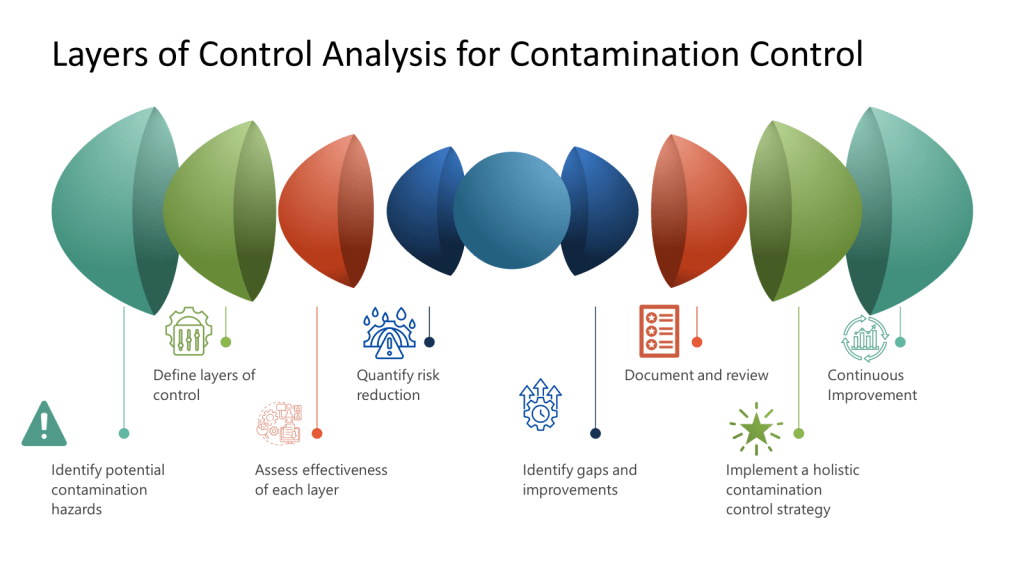
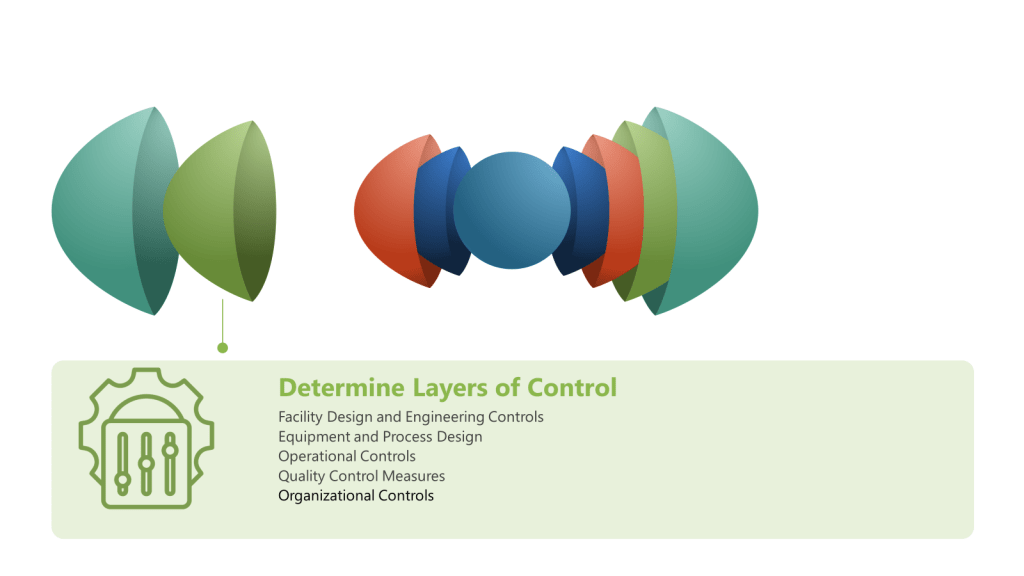
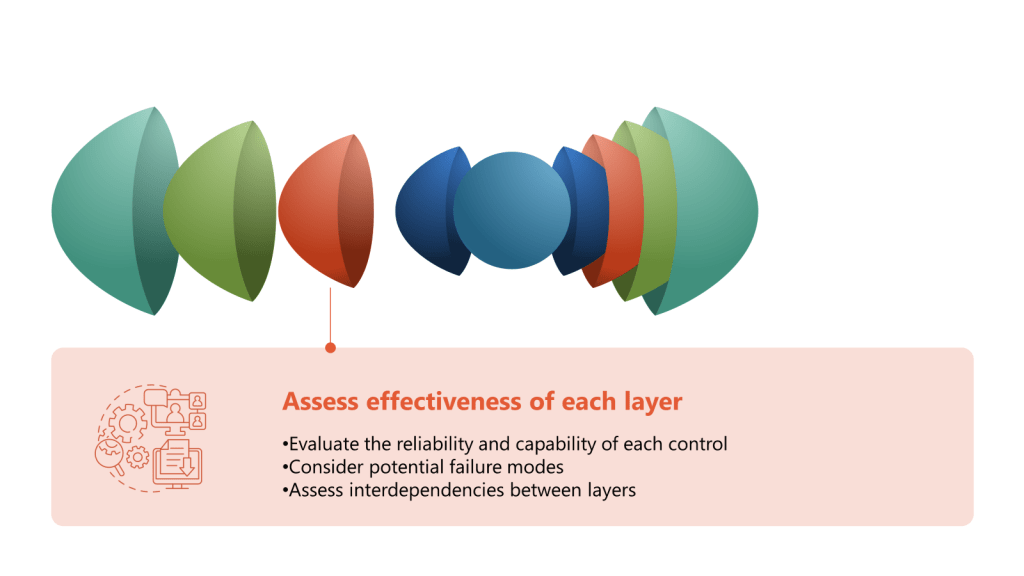
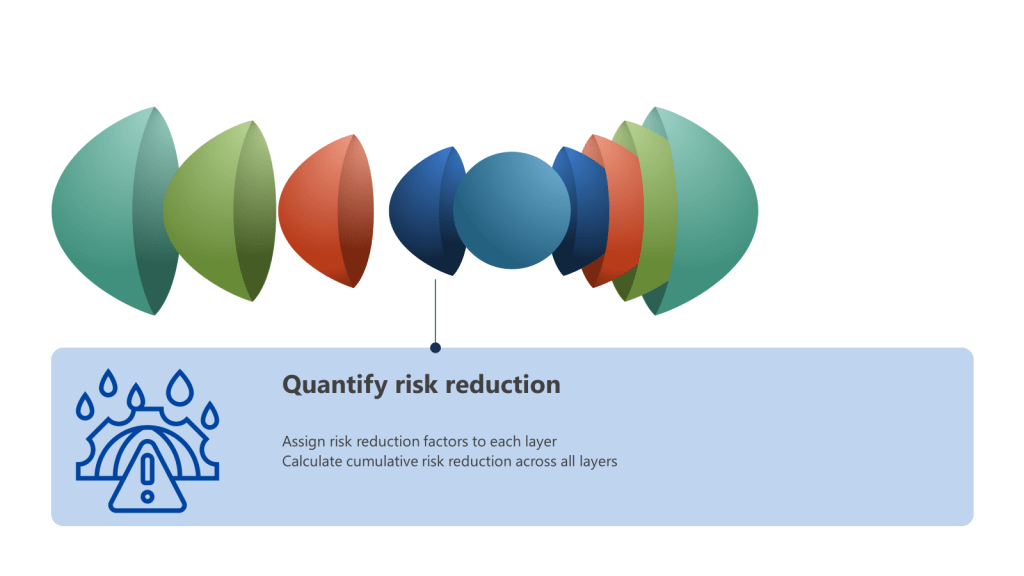
A Hazard and Operability Study (HAZOP) is a structured and systematic examination of a complex planned or existing process or operation to identify and evaluate problems that may represent risks to product, personnel or equipment. The primary goal of a HAZOP is to ensure that risks are managed effectively by identifying potential hazards and operability problems and developing appropriate mitigation strategies.
Why Use HAZOP?
Biotech facilities involve intricate processes that can be prone to various risks, including contamination, equipment failure, and process deviations. Implementing a HAZOP can:
- Risk Identification and Mitigation: HAZOPs help identify potential hazards associated with biotech processes, such as contamination risks, equipment malfunctions, and deviations from standard operating procedures. By identifying these risks, facilities can implement mitigation strategies to prevent accidents and ensure safety.
- Process Optimization: Through the systematic analysis of processes, HAZOPs can identify inefficiencies and areas for improvement, leading to optimized operations and enhanced productivity.
Part of a Continuum of Risk Tools
A HAZOP (Hazard and Operability) study differs from other risk assessment methods in a few key ways:
- Systematic examination of process deviations: HAZOP uses a very structured approach of examining potential deviations from the intended design and operation of a process, using guidewords like “more”, “less”, “no”, “reverse”, etc. This systematic approach helps identify hazards that may be missed by other methods.
- Focus on operability issues: The HAZOP examines operability problems that could impact process efficiency or product quality.
- Node-by-node analysis: The process is broken down into nodes or sections that are analyzed individually, allowing for very thorough examination.
- Qualitative analysis: Unlike quantitative risk assessment methods, HAZOP is primarily qualitative, focusing on identifying potential hazards rather than quantifying risk levels. HAZOPs do not typically assign numerical scores or rankings to risks.
- Consideration of causes and consequences: For each deviation, the team examines possible causes, consequences, and existing safeguards before recommending additional actions.
- Applicable to complex processes: The structured approach makes HAZOP well-suited for analyzing complex processes with many variables and potential interactions.
| Method | Description | Strengths | Limitations |
|---|---|---|---|
| HAZOP (Hazard and Operability Study) | Systematic examination of process/operation to identify potential hazards and operability problems | – Very thorough and structured approach – Examines deviations from design intent – Team-based | – Time consuming – Primarily qualitative |
| FMEA (Failure Mode and Effects Analysis) | Systematic method to identify potential failure modes and their effects | – Quantitative risk prioritization – Proactive approach – Can be used on products and processes | – Does not consider combinations of failures – Can be subjective |
| HACCP (Hazard Analysis and Critical Control Points) | Systematic approach to food safety hazards | – Focus on prevention – Identifies critical control points | – Requires prerequisite programs in place |
| PHA (Preliminary Hazard Analysis) | Early stage hazard identification technique | – Can be used early in design process – Relatively quick to perform – Identifies major hazards | – Not very detailed – Qualitative only – May miss some hazards |
| Bow-Tie Analysis | Combines fault tree and event tree analysis | – Visual representation of risk pathways – Shows preventive and mitigative controls – Good communication tool | – Does not show detailed failure logic – Can oversimplify complex scenarios – Time consuming for multiple hazards |
Key differences:
- HAZOP focuses on deviations from design intent, while FMEA looks at potential failure modes
- HACCP is specific to identify hazards and is commonly used in food safety, while the others are more general risk assessment tools
- PHA is used early in design, while the others are typically used on existing systems
- Bow-Tie provides a visual risk pathway, while the others use more tabular formats
- FMEA and HAZOP tend to be the most thorough and time-intensive methods
The choice of method depends on the specific application, stage of design, and level of detail required. Often a combination of methods may be used.
Instructions for Conducting a HAZOP
Preparation
- Assemble a multidisciplinary team comprising appropriate experts
- Define the scope of the HAZOP study, including the specific processes or operations to be analyzed.
- Gather and review all relevant documentation, such as process flow diagrams, piping and instrumentation diagrams, and standard operating procedures.
Execution
- Divide the Process into Nodes: Break down the process into manageable sections or nodes. Each node typically represents a specific part of the process, such as a piece of equipment or a process step.
- Identify Deviations: For each node, guidewords are applied to identify potential deviations from the intended design or operation. Common guidewords include:
- No: Complete absence of a process parameter (e.g., no flow).
- More: Quantitative increase (e.g., more pressure).
- Less: Quantitative decrease (e.g., less temperature).
- As well as: Presence of additional elements (e.g., contamination).
- Part of: Partial completion of an action (e.g., partial mixing).
- Reverse: Logical opposite of the intended action (e.g., reverse flow).
- Analyze Causes and Consequences: Determine the possible causes of each deviation and analyze the potential consequences on safety, environment, and operations. This involves considering various factors such as equipment failure, human error, environmental conditions, or procedural issues that could lead to the deviation.
- Use of Experience and Knowledge: The team relies on their collective experience and knowledge of the process, equipment, and industry standards to hypothesize potential causes. This may include reviewing historical data, previous incidents, and near misses.
- Recommend Actions: Develop recommendations for mitigating identified risks, such as changes to the process, additional controls, or procedural modifications.
Documentation and Follow-Up
- Document all findings, including identified hazards, potential consequences, and recommended actions.
- Assign responsibilities for implementing recommendations and establish timelines for completion.
- Conduct follow-up reviews to ensure that recommended actions have been implemented effectively and that the process remains safe and operable.
Review and Update
- Regularly review and update the HAZOP study to account for changes in processes, equipment, or regulations.
- Ensure continuous improvement by incorporating lessons learned from past incidents or near misses.
- Iterative Process: The process is iterative, with the team revisiting and refining their analysis as more information becomes available or as the understanding of the process deepens.
| Node | Guideword | Parameter | Deviation | Cause | Consequence | Safeguards | Recommendations | Actions |
|---|---|---|---|---|---|---|---|---|
| Specific section or equipment being analyzed | Guideword applied (e.g. No, More, Less, Reverse, etc.) | Process parameter being examined (e.g. Flow, Temperature, Pressure, etc.) | How the parameter deviates from design intent when guideword is applied | Possible reasons for the deviation | Potential results if deviation occurs | Existing measures to prevent or mitigate the deviation | Suggested additional measures to control the risk | Specific tasks assigned to implement recommendations |