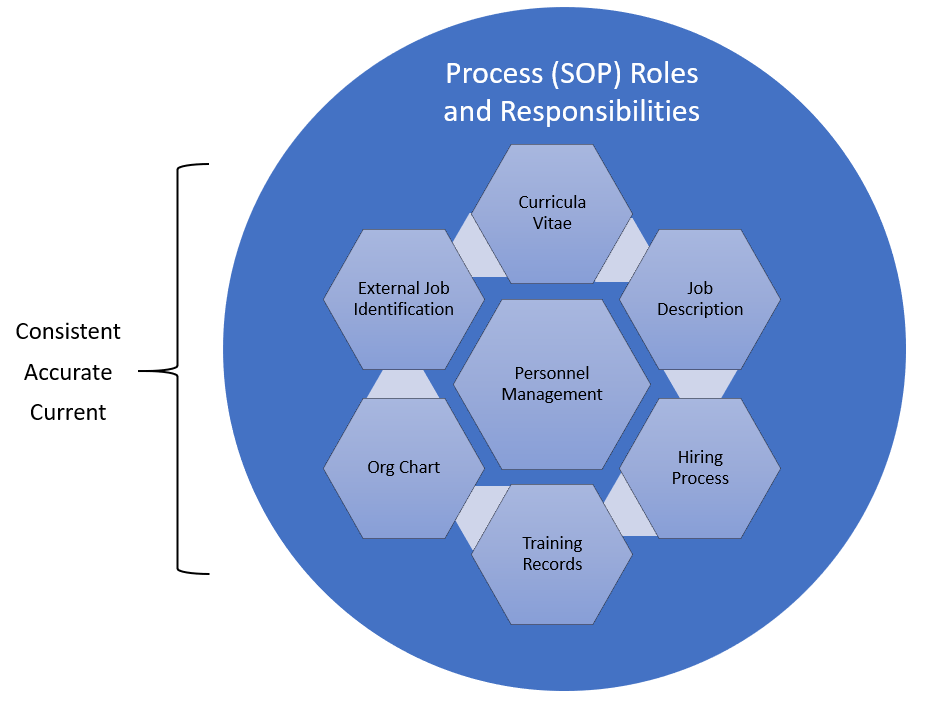
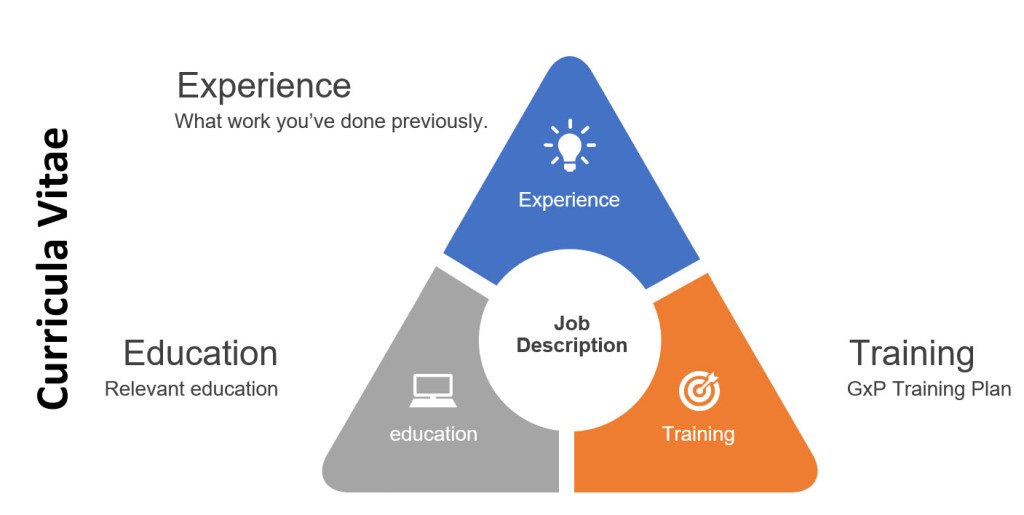
Leveraging the company’s new employee orientation program can help both compliance and quality by ensuring that a new hire (or transfer) understands the expectations for employee performance which:
- Are covered by the GxP regulations, corporate policies and process/procedure
- Are written and readily available to employees
- Are mandatory
In a heavily regulated industry, like pharmaceuticals, this is especially important because an individual may not have experienced such regulation in their former position. Even within a regulated organization the level of regulatory experience will change as you move from one area to the next.
The new hire orientation process is not a once-and-done and should be long enough to truly make an impact on the individual’s performance.
This new hire program is seeing to engage new hires more rapidly within the quality culture to ensure that employee’s behavior aligns more rapidly with quality culture.
For example, the new hire orientation should reinforce that a pharmaceutical company is a regulated environment, responsible for products that can directly affect customers’ health and quality of life. Product failure could result in death or sickness. Working for an organization where products help preserve and sustain life comes with the responsibility to know one’s job and perform it correctly at all time.
New hire orientation must present the organization’s cultural imperative for quality – why is it important to fulfill the organization’s purpose or reason for being – and how has this been embedded into the organization’s culture. At heart new hire orientation should answer three questions:
- What does quality mean to you personally and how do you exhibit in the organization?
- What are the expectations for quality outcomes in the organization and how we judge that they have been met.
- How quality is embedded in the culture and daily work of the people in this organization and what represents good role model behaviors.
Content that does not immediately impact the new hire, or only impacts new hires in several departments or units, is better deferred until later training activities.
Topics that would be in that immediate review include:
- What does it mean to work in a regulated environment
- The role of the quality system
- How to access process/procedure and complete training
- Good documentation practices and data integrity (high level)
- How to engage with regulatory stakeholders and other external partners (high level)
What other material would you cover?