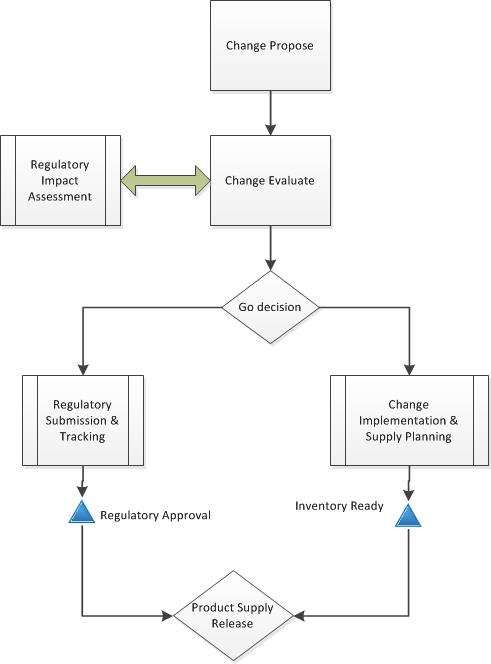
A colleague asks “How do you manage changes and disposition when doing long-term validation or specification setting in multiple markets?”

Perhaps it is a cleaning validation or a major process change or a new filter or raw material. You need to be able to disposition products against a change control to some markets but not all.
It is important to realize that changes become effective multiple times. Looking back on the post “Changes become Effective” we are managing after change-in-use where the regulatory approval is not being simultaneously gated and product will be sent to market on a case-by-case basis.
The change control contains a corresponding regulatory assessment with required variations for all impacted markets and a disposition strategy aligned with validation activities. With action items (e.g. work, tasks) in the change control for ongoing evaluation of lots impacted by study.
That disposition strategy might include evaluation of data vs acceptance criteria for each lot via checklist (or another tool) included in disposition packet, or an evaluation of data through change control task and disposition references change control. They are pretty similar in results and it more ends up being a record management preference.