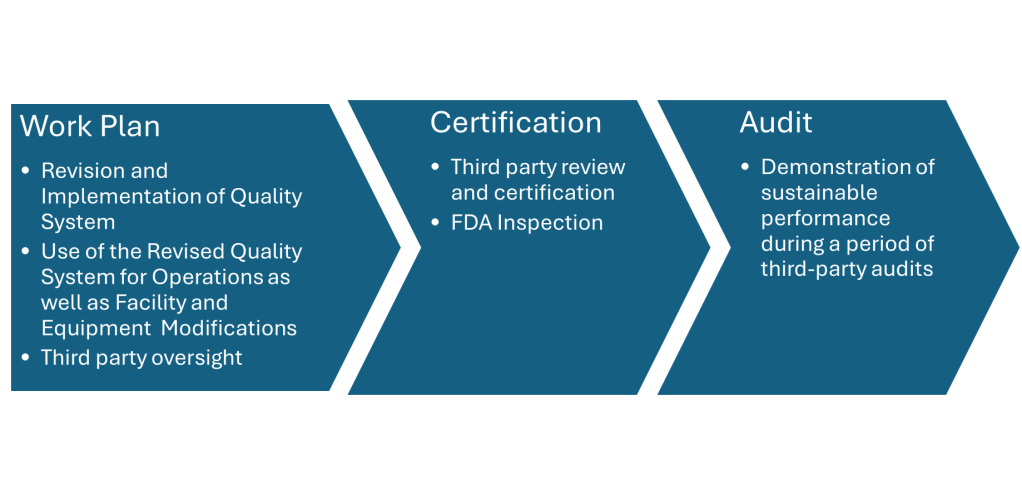
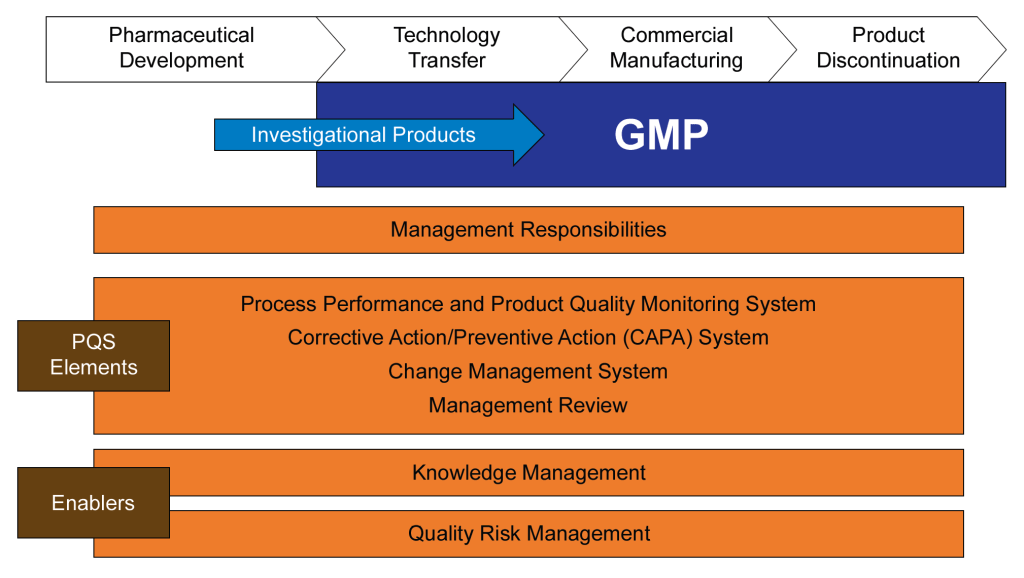
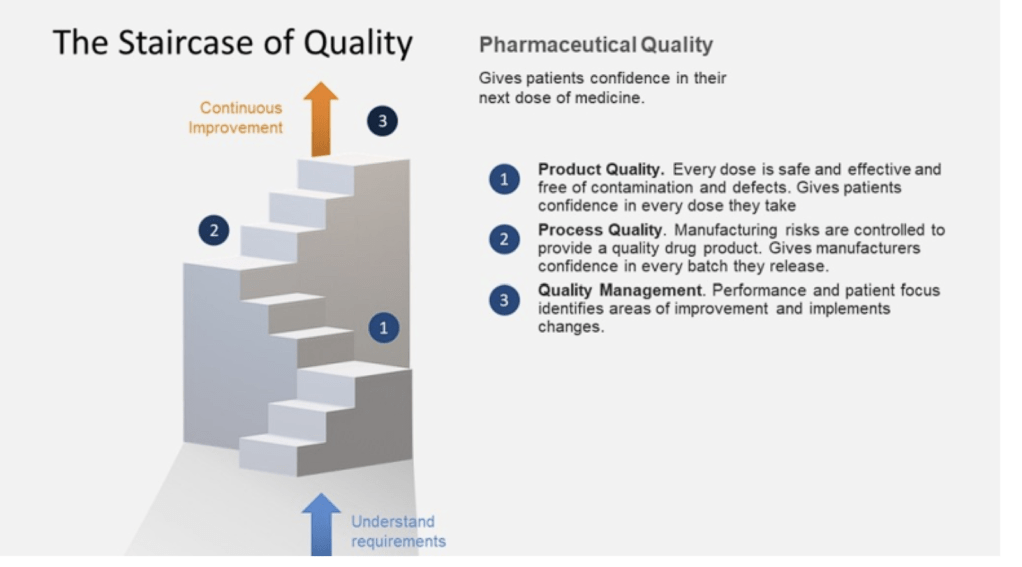
In the ever-evolving landscape of pharmaceutical manufacturing, quality management has become a cornerstone of success. Two key frameworks guiding this pursuit of excellence are the ICH Q10 Pharmaceutical Quality System and the FDA’s Quality Management Maturity (QMM) program. At the heart of these initiatives lies the quality plan – a crucial document that outlines an organization’s approach to ensuring consistent product quality and continuous improvement.
What is a Quality Plan?
A quality plan serves as a roadmap for achieving quality objectives and ensuring that all stakeholders are aligned in their pursuit of excellence.
Key components of a quality plan typically include:
- Organizational objectives to drive quality
- Steps involved in the processes
- Allocation of resources, responsibilities, and authority
- Specific documented standards, procedures, and instructions
- Testing, inspection, and audit programs
- Methods for measuring achievement of quality objectives
Aligning with ICH Q10 Management Responsibilities
ICH Q10 provides a model for an effective pharmaceutical quality system that goes beyond the basic requirements of Good Manufacturing Practice (GMP). To meet ICH Q10 management responsibilities, a quality plan should address the following areas:
1. Management Commitment
The quality plan should clearly articulate top management’s commitment to quality. This includes allocating necessary resources, participating in quality system oversight, and fostering a culture of quality throughout the organization.
2. Quality Policy and Objectives
Align your quality plan with your organization’s overall quality policy. Define specific, measurable quality objectives that support the broader goals of quality realization, establishing and maintaining a state of control, and facilitating continual improvement.
3. Planning
Outline the strategic approach to quality management, including how quality considerations are integrated into product lifecycle stages from development through to discontinuation.
4. Resource Management
Detail how resources (human, financial, and infrastructural) will be allocated to support quality initiatives. This includes provisions for training and competency development of personnel.
5. Management Review
Establish a process for regular management review of the quality system’s performance. This should include assessing the need for changes to the quality policy, objectives, and other elements of the quality system.
Aligning with FDA’s Quality Management Maturity Model
The FDA’s QMM program aims to encourage pharmaceutical manufacturers to go beyond basic compliance and foster a culture of quality and continuous improvement. To align your quality plan with QMM principles, consider incorporating the following elements:
1. Quality Culture
Describe how your organization will foster a strong quality culture mindset. This includes promoting open communication, encouraging employee engagement in quality initiatives, and recognizing quality-focused behaviors.
2. Continuous Improvement
Detail processes for identifying areas where quality management practices can be enhanced. This might include regular assessments, benchmarking against industry best practices, and implementing improvement projects.
3. Risk Management
Outline a proactive approach to risk management that goes beyond basic compliance. This should include processes for identifying, assessing, and mitigating risks to product quality and supply chain reliability.
4. Performance Metrics
Define key performance indicators (KPIs) that will be used to measure and monitor quality performance. These metrics should align with the FDA’s focus on product quality, patient safety, and supply chain reliability.
5. Knowledge Management
Describe systems and processes for capturing, sharing, and utilizing knowledge gained throughout the product lifecycle. This supports informed decision-making and continuous improvement.
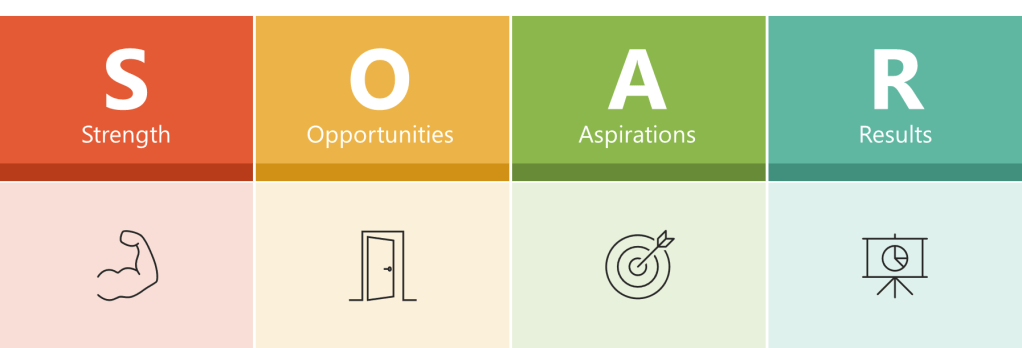
The SOAR Analysis
A SOAR Analysis is a strategic planning framework that focuses on an organization’s positive aspects and future potential. The acronym SOAR stands for Strengths, Opportunities, Aspirations, and Results.
Key Components
- Strengths: This quadrant identifies what the organization excels at, its assets, capabilities, and greatest accomplishments.
- Opportunities: This section explores external circumstances, potential for growth, and how challenges can be reframed as opportunities.
- Aspirations: This part focuses on the organization’s vision for the future, dreams, and what it aspires to achieve.
- Results: This quadrant outlines the measurable outcomes that will indicate success in achieving the organization’s aspirations.
Characteristics and Benefits
- Positive Focus: Unlike SWOT analysis, SOAR emphasizes strengths and opportunities rather than weaknesses and threats.
- Collaborative Approach: It engages stakeholders at all levels of the organization, promoting a shared vision.
- Action-Oriented: SOAR is designed to guide constructive conversations and lead to actionable strategies.
- Future-Focused: While addressing current strengths and opportunities, SOAR also projects a vision for the future.
Application
SOAR analysis is typically conducted through team brainstorming sessions and visualized using a 2×2 matrix. It can be applied to various contexts, including business strategy, personal development, and organizational change.
By leveraging existing strengths and opportunities to pursue shared aspirations and measurable results, SOAR analysis provides a framework for positive organizational growth and strategic planning.
The SOAR Analysis for Quality Plan Writing
Utilizing a SOAR (Strengths, Opportunities, Aspirations, Results) analysis can be an effective approach to drive the writing of a quality plan. This strategic planning tool focuses on positive aspects and future potential, making it particularly useful for developing a forward-looking quality plan. Here’s how you can leverage SOAR analysis in this process:
Conducting the SOAR Analysis
Strengths
Begin by identifying your organization’s current strengths related to quality. Consider:
- Areas where your organization excels in quality management
- Significant quality-related accomplishments
- Unique quality offerings that set you apart from competitors
Ask questions like:
- What are our greatest quality-related assets and capabilities?
- Where do we consistently meet or exceed quality standards?
Opportunities
Next, explore external opportunities that could enhance your quality initiatives. Look for:
- Emerging technologies that could improve quality processes
- Market trends that emphasize quality
- Potential partnerships or collaborations to boost quality efforts
Consider:
- How can we leverage external circumstances to improve our quality?
- What new skills or resources could elevate our quality standards?
Aspirations
Envision your preferred future state for quality in your organization. This step involves:
- Defining what you want to be known for in terms of quality
- Aligning quality goals with overall organizational vision
Ask:
- What is our ideal quality scenario?
- How can we integrate quality excellence into our long-term strategy?
Results
Finally, determine measurable outcomes that will indicate success in your quality initiatives. This includes:
- Specific, quantifiable quality metrics
- Key performance indicators (KPIs) for quality improvement
- Key behavior indicators (KBIs) and Key risk indicators (KRIs)
Consider:
- How will we measure progress towards our quality goals?
- What tangible results will demonstrate our quality aspirations have been achieved?
Writing the Quality Plan
With the SOAR analysis complete, use the insights gained to craft your quality plan:
- Executive Summary: Provide an overview of your quality vision, highlighting key strengths and opportunities identified in the SOAR analysis.
- Quality Objectives: Translate your aspirations into concrete, measurable objectives. Ensure these align with the strengths and opportunities identified.
- Strategic Initiatives: Develop action plans that leverage your strengths to capitalize on opportunities and achieve your quality aspirations. For each initiative, specify:
- Resources required
- Timeline for implementation
- Responsible parties
- Performance Metrics: Establish a system for tracking the results identified in your SOAR analysis. Include both leading and lagging indicators of quality performance.
- Continuous Improvement: Outline processes for regular review and refinement of the quality plan, incorporating feedback and new insights as they emerge.
- Resource Allocation: Based on the strengths and opportunities identified, detail how resources will be allocated to support quality initiatives.
- Training and Development: Address any skill gaps identified during the SOAR analysis, outlining plans for employee training and development in quality-related areas.
- Risk Management: While SOAR focuses on positives, acknowledge potential challenges and outline strategies to mitigate risks to quality objectives.
By utilizing the SOAR analysis framework, your quality plan will be grounded in your organization’s strengths, aligned with external opportunities, inspired by aspirational goals, and focused on measurable results. This approach ensures a positive, forward-looking quality strategy that engages stakeholders and drives continuous improvement.
A well-crafted quality plan serves as a bridge between regulatory requirements, industry best practices, and an organization’s specific quality goals. By aligning your quality plan with ICH Q10 management responsibilities and the FDA’s Quality Management Maturity model, you create a robust framework for ensuring product quality, fostering continuous improvement, and building a resilient, quality-focused organization.