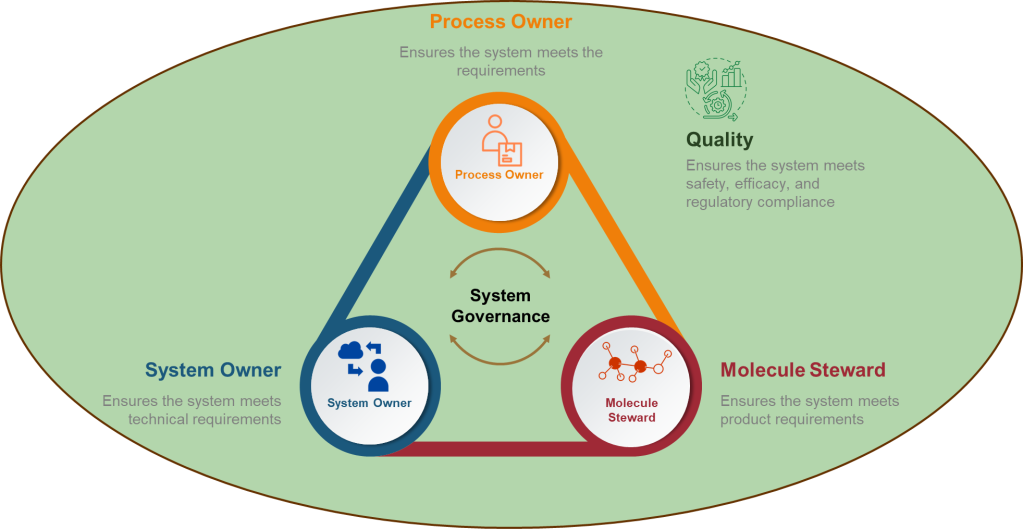
Defining the accountable individuals in a process is critical. In GAMP5, the technical System Owner role is distinct from the business Process Owner role, which focuses more on the system’s business process and compliance aspects.
The System Owner
The System Owner is responsible for the computerized system’s availability, support, and maintenance throughout its lifecycle. The System owner is the technical side of the equation and is often an IT director/manager or application support manager. Key responsibilities include:
- Defining, reviewing, approving, and implementing risk mitigation plans
- Ensuring technical requirements are documented
- Managing change control for the system
- Conducting evaluations for change requests impacting security, maintainability, data integrity, and architecture
- Performing system administration tasks like user and privilege maintenance
- Handling system patching, documentation of issues, and facilitating vendor support
Frankly, I think too many organizations make the system owner too low level. These lower-level individuals may perform system admin tasks and handle systems patching, but the more significant risk questions require extensive experience.
The System Owner focuses on the technical aspects of validation and ensures adequate procedural controls are in place after validation to maintain the validated state and protect data integrity.
The system owner requires learning and understanding new products and complex system architectures. They are the architect and need to be in charge of the big picture.
The Process Owner
In the context of GAMP5, a Process Owner plays a crucial role in the lifecycle management of computerized systems used in regulated industries such as pharmaceuticals and biotechnology. The Process Owner is ultimately accountable for the system’s implementation, validation, and ongoing compliant use.
I’ve written a lot about Process Owners. This use of process owner is 100% aligned with previous thinking.
Key Responsibilities of a Process Owner
- System Implementation and Validation: The Process Owner ensures the system is implemented and validated according to regulatory requirements and company policies. This includes overseeing the creation and maintenance of validation documentation and ensuring the system meets its intended use.
- Ongoing Compliance and Maintenance: The Process Owner must ensure the system remains validated throughout its lifecycle. This involves regular reviews, updates, and maintenance activities to ensure continued compliance with regulatory standards.
- Data Integrity and Quality: As the data owner maintains the system, the Process Owner is responsible for its integrity, administration, operation, maintenance, and decommissioning. They must ensure that data integrity and quality requirements are met and maintained.
- Decision-Making Authority: The Process Owner should be at a level within the organization that allows them to make business and process decisions regarding the system. This often includes roles such as operations director/manager, lab manager, or production manager.
- Collaboration with Other Teams: The Process Owner must collaborate with various teams, including Quality (QA), IT, Computer System Validation (CSV), training, HR, system vendors, and system development teams, to ensure that all necessary compliance activities are performed and documented promptly.
Skills and Knowledge Required
- Detailed Understanding of the System: The Process Owner should have a comprehensive understanding of the system, its purpose, functions, and use within the organization.
- Regulatory Knowledge: A good grasp of regulatory requirements is crucial for ensuring the system complies with all relevant guidelines and standards.
- Validation Practices: The Process Owner will sign off on validation documents and ensure that the system is fit for its intended use.
Comparison with the Molecule Steward
While the Molecule Steward, the ASTM E2500 SME role, is not directly equivalent to the GAMP 5 roles, it shares some similarities with both the system owner and process owner, particularly in terms of specialized knowledge and involvement in critical aspects of the system. It’s best to think of the Molecule Steward as the third part of this triad, ensuring the robustness of the scientific approach.
| System Owner | Process Owner | Molecule Steward | |
|---|---|---|---|
| Primary Focus | Technical aspects and maintenance of the system | Business process and compliance aspects | Specialized knowledge of critical aspects |
| Typical Role | IT director/manager or application support manager | Head of functional unit or department using the system | Subject matter expert in specific field |
| Key Responsibilities | – System availability, support, and maintenance – Data security – Risk mitigation plans – Technical requirements documentation – Change control management – Evaluating change requests | – Overall system integrity and compliance – Data ownership – User requirements definition – SOP development and maintenance – Ensuring GxP compliance – Approving key documentation – User training | – Defining system needs – Identifying critical aspects – Leading quality risk management – Developing verification strategies – Reviewing system designs – Executing verification tests |
| Expertise | Strong technical background | Business process knowledge | Specialized technical knowledge |
| Accountability | System performance and security | Business use and regulatory compliance | Critical aspects impacting product quality and patient safety |
| Involvement in Validation | Focuses on technical validation aspects | Ensures validation meets business needs | Leads verification activities |
Scale of the System
People make the system too small here. This isn’t equipment A or computer system X. It’s the entire system that produces result Y. For example, it is the manufacturing process for DS (or upstream DS), not the individual bioreactors. Lower-level assistants can help with wrangling, but there should be overall accountability. The system, process, and ASTM E2500 SME must have the power in the organization to be truly accountable.
The Role of Quality
The Quality Unit is responsible for ensuring the right process and procedure are in place, that regulatory requirements are met, and that the system is fit for use and fit for purpose. The Quality Unit in GAMP5 is crucial for ensuring the safety, efficacy, and regulatory compliance of pharmaceutical products and computerized systems.
- Ensuring Compliance and Product Quality: Quality is vital in ensuring that computerized systems used in pharmaceutical manufacturing meet regulatory requirements and consistently produce high-quality products. The Quality Unit helps organizations maintain high-quality standards in the various processes.
- Risk Management: The Quality Unit champions a science-based risk management approach to system validation and qualification. Quality ensures the identification and assessment of potential risks.
- Lifecycle Approach: The Quality Unit ensures that validation activities are conducted throughout the system’s lifecycle, from concept to retirement.
- Documentation and Traceability: The Quality Unit oversees comprehensive documentation and traceability throughout the system’s lifecycle. Detailed records enable transparency, facilitate audits, and demonstrate compliance with regulatory requirements.
- Change Management: The Quality Unit evaluates and controls system changes to ensure that modifications do not compromise product quality or patient safety.
- Data Integrity: Quality is crucial in maintaining data integrity and ensuring records’ accuracy, reliability, and completeness.
- Supplier and Internal Audits: Quality regularly audits suppliers and internal processes to ensure compliance and quality. These audits help identify gaps and areas for improvement in system development, implementation, and maintenance.
Beyond GAMP5
I consider this the best practice for handling an ASTM E2500 approach.