In the highly regulated pharmaceutical industry, ensuring the quality, safety, and efficacy of products is paramount. Two critical components of pharmaceutical quality management are Quality Assurance (QA) and Quality Control (QC). While these terms are sometimes used interchangeably, they represent distinct approaches with different focuses, methodologies, and objectives within pharmaceutical manufacturing. Understanding the differences between QA and QC is essential for pharmaceutical companies to effectively manage their quality processes and meet regulatory requirements.
Quality Assurance (QA) and Quality Control (QC) are both essential and complementary pillars of pharmaceutical quality management, each playing a distinct yet interconnected role in ensuring product safety, efficacy, and regulatory compliance. QA establishes the systems, procedures, and preventive measures that form the foundation for consistent quality throughout the manufacturing process, while QC verifies the effectiveness of these systems by testing and inspecting products to ensure they meet established standards. The synergy between QA and QC creates a robust feedback loop: QC identifies deviations or defects through analytical testing, and QA uses this information to drive process improvements, update protocols, and implement corrective and preventive actions. This collaboration not only helps prevent the release of substandard products but also fosters a culture of continuous improvement, risk mitigation, and regulatory compliance, making both QA and QC indispensable for maintaining the highest standards in pharmaceutical manufacturing.
Definition and Scope
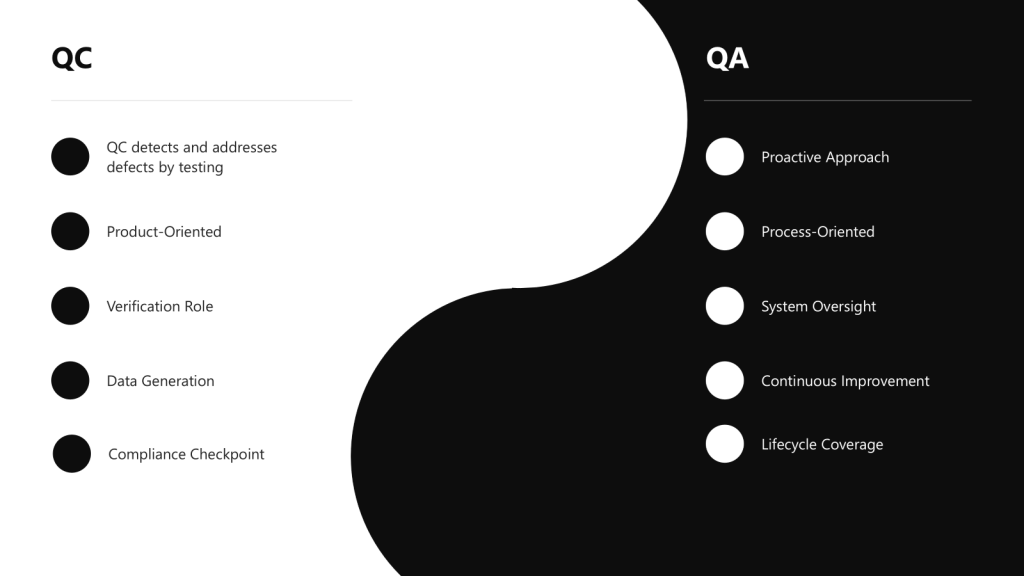
Quality Assurance (QA) is a comprehensive, proactive approach focused on preventing defects by establishing robust systems and processes throughout the entire product lifecycle. It encompasses the totality of arrangements made to ensure pharmaceutical products meet the quality required for their intended use. QA is process-oriented and aims to build quality into every stage of development and manufacturing.
Quality Control (QC) is a reactive, product-oriented approach that involves testing, inspection, and verification of finished products to detect and address defects or deviations from established standards. QC serves as a checkpoint to identify any issues that may have slipped through the manufacturing process.
Approach: Proactive vs. Reactive
One of the most fundamental differences between QA and QC lies in their approach to quality management:
- QA takes a proactive approach by focusing on preventing defects and deviations before they occur. It establishes robust quality management systems, procedures, and processes to minimize the risk of quality issues.
- QC takes a reactive approach by focusing on detecting and addressing deviations and defects after they have occurred. It involves testing, sampling, and inspection activities to identify non-conformities and ensure products meet established quality standards.
Focus: Process vs. Product
- QA is process-oriented, focusing on establishing and maintaining robust processes and procedures to ensure consistent product quality. It involves developing standard operating procedures (SOPs), documentation, and validation protocols.
- QC is product-oriented, focusing on verifying the quality of finished products through testing and inspection. It ensures that the final product meets predetermined specifications before release to the market.
Comparison Table: QA vs. QC in Pharmaceutical Manufacturing
| Aspect | Quality Assurance (QA) | Quality Control (QC) |
|---|---|---|
| Definition | A comprehensive, proactive approach focused on preventing defects by establishing robust systems and processes | A reactive, product-oriented approach that involves testing and verification of finished products |
| Focus | Process-oriented, focusing on how products are made | Product-oriented, focusing on what is produced |
| Approach | Proactive – prevents defects before they occur | Reactive – detects defects after they occur |
| Timing | Before and during production | During and after production |
| Responsibility | Establishing systems, procedures, and documentation | Testing, inspection, and verification of products This includes the appropriate control of analytical methods. |
| Activities | System development, documentation, risk management, training, audits, supplier management, change control, validation | Raw materials testing, in-process testing, finished product testing, dissolution testing, stability testing, microbiological testing |
| Objective | To build quality into every stage of development and manufacturing | To identify non-conformities and ensure products meet specifications |
| Methodology | Establishing SOPs, validation protocols, and quality management systems | Sampling, testing, inspection, and verification activities |
| Scope | Spans the entire product lifecycle from development to discontinuation | Primarily focused on manufacturing and finished products |
| Relationship to GMP | Ensures GMP implementation through systems and processes | Verifies GMP compliance through testing and inspection |
The Quality Continuum: QA and QC as Complementary Approaches
Rather than viewing QA and QC as separate entities, modern pharmaceutical quality systems recognize them as part of a continuous spectrum of quality management activities. This continuum spans the entire product lifecycle, from development through manufacturing to post-market surveillance.
The Integrated Quality Approach
QA and QC represent different points on the quality continuum but work together to ensure comprehensive quality management. The overlap between QA and QC creates an integrated quality approach where both preventive and detective measures work in harmony. This integration is essential for maintaining what regulators call a “state of control” – a condition in which the set of controls consistently provides assurance of continued process performance and product quality.
Quality Risk Management as a Bridge
Quality Risk Management (QRM) serves as a bridge between QA and QC activities, providing a systematic approach to quality decision-making. By identifying, assessing, and controlling risks throughout the product lifecycle, QRM helps determine where QA preventive measures and QC detective measures should be applied most effectively.
The concept of a “criticality continuum” further illustrates how QA and QC work together. Rather than categorizing quality attributes and process parameters as simply critical or non-critical, this approach recognizes varying degrees of criticality that require different levels of control and monitoring.
Organizational Models for QA and QC in Pharmaceutical Companies
Pharmaceutical companies employ various organizational structures to manage their quality functions. The choice of structure depends on factors such as company size, product portfolio complexity, regulatory requirements, and corporate culture.
Common Organizational Models
Integrated Quality Unit
In this model, QA and QC functions are combined under a single Quality Unit with shared leadership and resources. This approach promotes streamlined communication and a unified approach to quality management. However, it may present challenges related to potential conflicts of interest and lack of independent verification.
Separate QA and QC Departments
Many pharmaceutical companies maintain separate QA and QC departments, each with distinct leadership reporting to a higher-level quality executive. This structure provides clear separation of responsibilities and specialized focus but may create communication barriers and resource inefficiencies.
QA as a Standalone Department, QC Integrated with Operations
In this organizational model, the Quality Assurance (QA) function operates as an independent department, while Quality Control (QC) is grouped within the same department as other operations functions, such as manufacturing and production. This structure is designed to balance independent oversight with operational efficiency.
Centralized Quality Organization
Large pharmaceutical companies often adopt a centralized quality organization where quality functions are consolidated at the corporate level with standardized processes across all manufacturing sites. This model ensures consistent quality standards and efficient knowledge sharing but may be less adaptable to site-specific needs.
Decentralized Quality Organization
In contrast, some companies distribute quality functions across manufacturing sites with site-specific quality teams. This approach allows for site-specific quality focus and faster decision-making but may lead to inconsistent quality practices and regulatory compliance challenges.
Matrix Quality Organization
A matrix quality organization combines elements of both centralized and decentralized models. Quality personnel report to both functional quality leaders and operational/site leaders, providing a balance between standardization and site-specific needs. However, this structure can create complex reporting relationships and potential conflicts in priorities.
The Quality Unit: Overarching Responsibility for Pharmaceutical Quality
Concept and Definition of the Quality Unit
The Quality Unit is a fundamental concept in pharmaceutical manufacturing, representing the organizational entity responsible for overseeing all quality-related activities. According to FDA guidance, the Quality Unit is “any person or organizational element designated by the firm to be responsible for the duties relating to quality control”.
The concept of a Quality Unit was solidified in FDA’s 2006 guidance, “Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations,” which defined it as the entity responsible for creating, monitoring, and implementing a quality system.
Independence and Authority of the Quality Unit
Regulatory agencies emphasize that the Quality Unit must maintain independence from production operations to ensure objective quality oversight. This independence is critical for the Quality Unit to fulfill its responsibility of approving or rejecting materials, processes, and products without undue influence from production pressures.
The Quality Unit must have sufficient authority and resources to carry out its responsibilities effectively. This includes the authority to investigate quality issues, implement corrective actions, and make final decisions regarding product release.
How QA and QC Contribute to Environmental Monitoring and Contamination Control
Environmental monitoring (EM) and contamination control are critical pillars of pharmaceutical manufacturing quality systems, requiring the coordinated efforts of both Quality Assurance (QA) and Quality Control (QC) functions. While QA focuses on establishing preventive systems and procedures, QC provides the verification and testing that ensures these systems are effective. Together, they create a comprehensive framework for maintaining aseptic manufacturing environments and protecting product integrity. This also serves as a great example of the continuum in action.
QA Contributions to Environmental Monitoring and Contamination Control
System Design and Program Development
Quality Assurance takes the lead in establishing the foundational framework for environmental monitoring programs. QA is responsible for designing comprehensive EM programs that include sampling plans, alert and action limits, and risk-based monitoring locations. This involves developing a systematic approach that addresses all critical elements including types of monitoring methods, culture media and incubation conditions, frequency of environmental monitoring, and selection of sample sites.
For example, QA establishes the overall contamination control strategy (CCS) that defines and assesses the effectiveness of all critical control points, including design, procedural, technical, and organizational controls employed to manage contamination risks. This strategy encompasses the entire facility and provides a comprehensive framework for contamination prevention.
Risk Management and Assessment
QA implements quality risk management principles to provide a proactive means of identifying, scientifically evaluating, and controlling potential risks to quality. This involves conducting thorough risk assessments that cover all human interactions with clean room areas, equipment placement and ergonomics, and air quality considerations. The risk-based approach ensures that monitoring efforts are focused on the most critical areas and processes where contamination could have the greatest impact on product quality.
QA also establishes risk-based environmental monitoring programs that are re-evaluated at defined intervals to confirm effectiveness, considering factors such as facility aging, barrier and cleanroom design optimization, and personnel changes. This ongoing assessment ensures that the monitoring program remains relevant and effective as conditions change over time.
Procedural Oversight and Documentation
QA ensures the development and maintenance of standardized operating procedures (SOPs) for all aspects of environmental monitoring, including air sampling, surface sampling, and personnel sampling protocols. These procedures ensure consistency in monitoring activities and provide clear guidance for personnel conducting environmental monitoring tasks.
The documentation responsibilities of QA extend to creating comprehensive quality management plans that clearly define responsibilities and duties to ensure that environmental monitoring data generated are of the required type, quality, and quantity. This includes establishing procedures for data analysis, trending, investigative responses to action level excursions, and appropriate corrective and preventative actions.
Compliance Assurance and Regulatory Alignment
QA ensures that environmental monitoring protocols meet Good Manufacturing Practice (GMP) requirements and align with current regulatory expectations such as the EU Annex 1 guidelines.
QA also manages the overall quality system to ensure that environmental monitoring activities support regulatory compliance and facilitate successful inspections and audits. This involves maintaining proper documentation, training records, and quality improvement processes that demonstrate ongoing commitment to contamination control.
QC Contributions to Environmental Monitoring and Contamination Control
Execution of Testing and Sampling
Quality Control is responsible for the hands-on execution of environmental monitoring testing protocols. QC personnel conduct microbiological testing including bioburden and endotoxin testing, as well as particle counting for non-viable particulate monitoring. This includes performing microbial air sampling using techniques such as active air sampling and settle plates, along with surface and personnel sampling using swabbing and contact plates.
For example, QC technicians perform routine environmental monitoring of classified manufacturing and filling areas, conducting both routine and investigational sampling to assess environmental conditions. They utilize calibrated active air samplers and strategically placed settle plates throughout cleanrooms, while also conducting surface and personnel sampling periodically, especially after critical interventions.
Data Analysis and Trend Monitoring
QC plays a crucial role in analyzing environmental monitoring data and identifying trends that may indicate potential contamination issues. When alert or action limits are exceeded, QC personnel initiate immediate investigations and document findings according to established protocols. This includes performing regular trend analysis on collected data to understand the state of control in cleanrooms and identify potential contamination risks before they lead to significant problems.
QC also maintains environmental monitoring programs and ensures all data is properly logged into Laboratory Information Management Systems (LIMS) for comprehensive tracking and analysis . This systematic approach to data management enables effective trending and supports decision-making processes related to contamination control.
Validation and Verification Activities
QC conducts critical validation activities to simulate aseptic processes and verify the effectiveness of contamination control measures. These activities provide direct evidence that manufacturing processes maintain sterility and/or bioburden control and that environmental controls are functioning as intended.
QC also performs specific testing protocols including dissolution testing, stability testing, and comprehensive analysis of finished products to ensure they meet quality specifications and are free from contamination. This testing provides the verification that QA-established systems are effectively preventing contamination.
Real-Time Monitoring and Response
QC supports continuous monitoring efforts through the implementation of Process Analytical Technology (PAT) for real-time quality verification. This includes continuous monitoring of non-viable particulates, which helps detect events that could potentially increase contamination risk and enables immediate corrective measures.
When deviations occur, QC personnel immediately report findings and place products on hold for further evaluation, providing documented reports and track-and-trend data to support decision-making processes. This rapid response capability is essential for preventing contaminated products from reaching the market.
Conclusion
While Quality Assurance and Quality Control in pharmaceutical manufacturing represent distinct processes with different focuses and approaches, they form a complementary continuum that ensures product quality throughout the lifecycle. QA is proactive, process-oriented, and focused on preventing quality issues through robust systems and procedures. QC is reactive, product-oriented, and focused on detecting and addressing quality issues through testing and inspection.
The organizational structure of quality functions in pharmaceutical companies varies, with models ranging from integrated quality units to separate departments, centralized or decentralized organizations, and matrix structures. Regardless of the organizational model, the Quality Unit plays a critical role in overseeing all quality-related activities and ensuring compliance with regulatory requirements.
The Pharmaceutical Quality System provides an overarching framework that integrates QA and QC activities within a comprehensive approach to quality management. By implementing effective quality systems and fostering a culture of quality, pharmaceutical companies can ensure the safety, efficacy, and quality of their products while meeting regulatory requirements and continuously improving their processes.