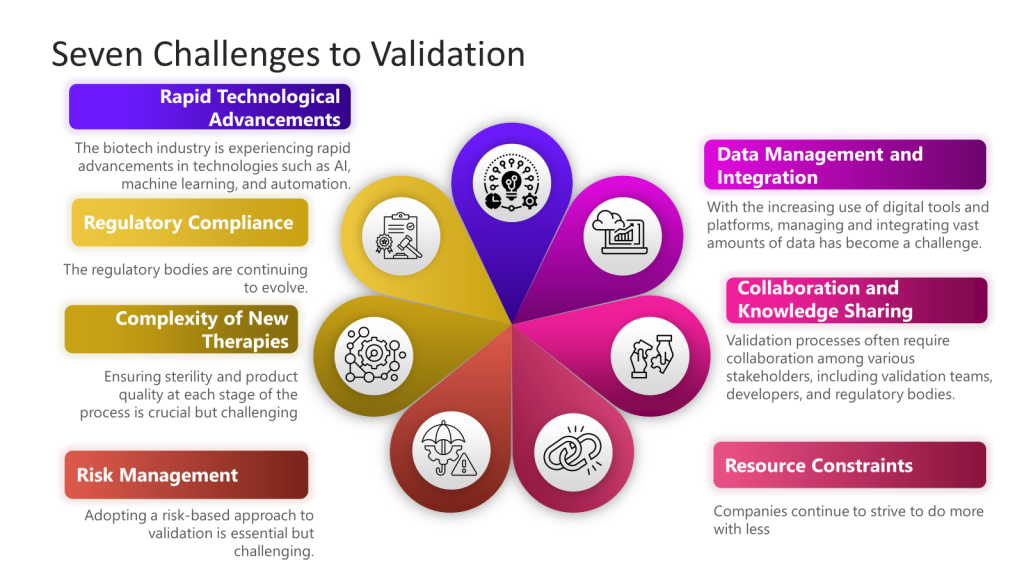
One of the topics I’m passionate about is exploring the changing landscape of quality management and the challenges we face. The solutions that worked in the past decade won’t be as effective in our current era, marked by post-globalization, capital rationalization, spatial dispersion, shrinking workforces, and an increasing reliance on automation. This transformation calls for a new perspective on quality management, as traditional instincts and strategies may no longer be sufficient. The nature of opportunity and risk has fundamentally changed, and in order to thrive, we need to adapt our approach.
The New Rules of Engagement
In this era of volatility, several key trends are reshaping the business environment:
- Post-Globalization: The shift towards localized operations and supply chains.
- Capital Rationalization: More stringent allocation of financial resources. This is a huge trend in biotech.
- Spatial Dispersion: Decentralized workforces and operations.
- Shrinking Workforces: Reduced human resources due to demographic changes.
- Dependence on Automation: Increased reliance on technologies like AI, ML, and RPA.
We need to reevaluate how we approach quality management in light of these trends.
Prediction: Anticipating the Future
In a volatile environment, it is crucial to predict and anticipate disruptions. Quality management must shift from being reactive to proactive. This involves:
- Advanced Analytics: Utilizing data analytics to anticipate quality issues before they emerge. This necessitates a strong data foundation and the capability to analyze both structured and unstructured data.
- Scenario Planning: Developing multiple scenarios to anticipate potential disruptions and their impacts on quality aids in making well-informed strategic decisions and preparing for various contingencies.
Adaptability: Embracing Change
Adaptability is crucial in a constantly changing world. Quality management systems need to be flexible and responsive to new challenges.
- Agile Methodologies: Implementing agile practices to allow for quick adjustments to processes and workflows, fostering a culture of experimentation, and learning from failures.
- Virtualization of Work: Adapting quality processes to support remote and hybrid work environments involves re-evaluating governance models and ensuring that quality standards are maintained regardless of the location of work.
Resilience: Building Robust Systems
Resilience ensures that organizations can withstand and recover from disruptions. This capability is built on strong foundations:
- Robust Systems: Developing systems that can operate effectively under stress. This includes ensuring that automated processes are reliable and that there are contingencies for system failures.
- Organizational Culture: Fostering a culture that values resilience and continuous improvement ensures that employees are prepared to handle disruptions and contribute to the organization’s long-term success.
Implementing the New Quality Paradigm
To effectively implement these principles, organizations should consider the following steps:
- Assess the Current State: Conduct a comprehensive assessment of existing quality processes, identifying areas for improvement and potential vulnerabilities.
- Set Clear Objectives: Establish clear, measurable objectives that align with the principles of prediction, adaptability, and resilience.
- Develop a Phased Approach: Implement changes gradually, with clear milestones and measurable outcomes to ensure smooth transitions.
- Engage Stakeholders: Involve all relevant stakeholders in the transformation process to ensure alignment and buy-in.
- Monitor Progress: Continuously monitor progress against predefined objectives and make adjustments as necessary to stay on track.
- Invest in Training: Provide employees with the necessary training and development opportunities to adapt to new technologies and processes.
Conclusion
It is important to change our mindset and strategy. Embracing the principles of prediction, adaptability, and resilience can help organizations navigate the complexities of a volatile environment and position themselves for long-term success. Going forward, it is essential to stay vigilant, flexible, and proactive in our approach to quality management. We must ensure that we not only meet but exceed stakeholder expectations in this rapidly changing world.