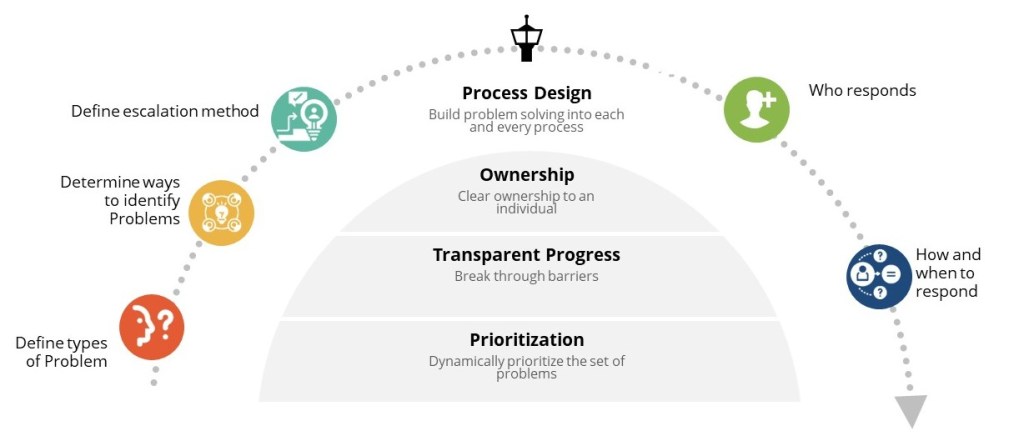
We tend to jumble forms of accountability in an organization, often confusing between a people manager and a technical manager. I think its very important to differentiate between the two.
People managers deal with human resources and team dynamics, while technical managers deal with managing design, execution, and improvement. They can be the same person, but we need to recognize the differences and resource appropriately. Too often we blur the two roles and as a result neither is done well.
I’ve talked on this blog about a few of the technical manager types: Process Owners, the ASTM E2500 SME/Molecule Steward, and Knowledge Owners. There are certainly others out there. In the table below I added two more for comparison:
- a qualified person from OSHA, because I think this is a great generic look at the concept
- The EU Qualifed Person. Industry relevant and one that often gets confused in execution.
| Aspect | Qualified Person (OSHA Definition) | Qualified Person (EU) | Knowledge Owner | ASTM E2500 SME | Process Owner |
|---|---|---|---|---|---|
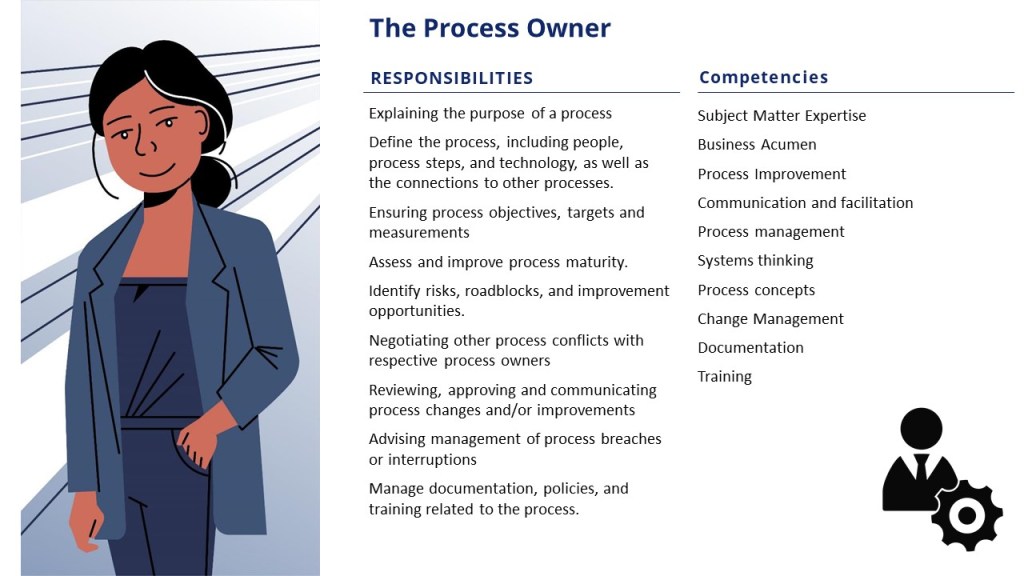
| Primary Focus | Ensuring compliance with safety standards and solving technical problems | Certifying that each batch of a medicinal product meets all required provisions | Managing and maintaining knowledge within a specific domain | Ensuring manufacturing systems meet quality and safety standards | Managing and optimizing a specific business process |
| Key Responsibilities | Solve or resolve problems related to the subject matter, work, or project | Certify batches meet GMP and regulatory standards | Maintain and update knowledge base | Define system needs and identify critical aspects | Define process goals, purpose, and KPIs |
| Design and install systems to improve safety | Ensure compliance with market authorization requirements | Validate and broadcast new knowledge | Develop and execute verification strategies | Communicate with key players and stakeholders | |
| Ensure compliance with laws and standards | Oversee quality control and assurance processes | Provide training and support | Review system designs and manage risks | Analyze process performance and identify improvements | |
| May not have the authority to stop work | Conduct audits and inspections | Monitor and update knowledge assets | Lead quality risk management efforts | Ensure process compliance with regulations and standards | |
| Skills Required | Technical expertise in the area | Degree in pharmacy, biology, chemistry, or related field | Subject matter expertise in specific knowledge domain | Technical understanding of manufacturing systems and equipment | Leadership and communication skills |
| Certification, degree, or other professional recognition | Several years of experience in pharmaceutical manufacturing | Analytical and validation skills | Risk management and verification skills | Analytical and problem-solving skills | |
| Ability to solve technical problems | Registered with the competent authority in the EU member state | Training and support skills | Continuous improvement and change management skills | Ability to define and monitor KPIs | |
| Authority | Authority to design and install safety systems | Authority to certify batches and ensure compliance | Authority over knowledge management processes and content | Authority to define and verify critical aspects of systems | Authority to make decisions and implement changes in the process |
| Interaction with Others | Collaborates with production and quality control teams | Works with quality control, assurance, and regulatory teams | Works with various departments to ensure knowledge is shared and utilized | Collaborates with project stakeholders and engineering teams | Communicates with project leaders, process users, and other stakeholders |
| Examples of Activities | Reviewing batch documentation and certifying products | Certifying each batch of medicinal products before release | Validating new knowledge submissions | Conducting quality risk analyses and verification tests | Defining process objectives and mission statements |
| Ensuring compliance with GMP and regulatory standards | Ensuring compliance with GMP and regulatory standards | Providing training on knowledge management systems | Reviewing system designs and managing changes | Monitoring process performance and compliance | |
| Overseeing investigations related to quality issues | Overseeing quality control and assurance processes | Updating and maintaining knowledge databases | Leading continuous improvement efforts | Identifying and implementing process improvements | |
| Industry Context | Primarily in construction, manufacturing, and safety-critical industries | Pharmaceutical and biotechnology industries within the EU | Applicable across various industries, especially information-heavy sectors | Primarily in pharmaceutical and biotechnology industries | Applicable in any industry with defined business processes |
- Qualified Person (OSHA Definition): Focuses on ensuring compliance with safety standards and solving technical problems. They possess technical expertise and professional recognition and are responsible for designing and installing safety systems.
- Qualified Person (EU): Ensures that each batch of medicinal products meets all required provisions before release. They are responsible for compliance with GMP and regulatory standards and must be registered with the competent authority in the EU member state.
- Knowledge Owner: Manages and disseminates knowledge within an organization. They ensure that knowledge is accurate, up-to-date, and accessible, and they provide training and support to facilitate knowledge sharing.
- ASTM E2500 SME: Ensures that manufacturing systems meet quality and safety standards. They define system needs, develop verification strategies, manage risks, and lead continuous improvement efforts.
- Process Owner: Manages and optimizes specific business processes. They define process goals, monitor performance, ensure compliance with standards, and implement improvements to enhance efficiency and effectiveness.
Common Themes
Subject Matter Expertise
- All roles require a high level of subject matter expertise in their respective domains, whether it’s technical knowledge, regulatory compliance, manufacturing processes, or business processes.
- This expertise is typically gained through formal education, certifications, extensive training, and practical experience.
Ensuring Compliance and Quality
- A key responsibility across these roles is ensuring compliance with relevant laws, regulations, standards, and quality requirements.
Risk Identification and Management
- These roles are all responsible for identifying potential risks, hazards, or process inefficiencies.
- They are expected to develop and implement strategies to mitigate or eliminate these risks, ensuring the safety of operations and the quality of products or processes.
Continuous Improvement and Change Management
- They are involved in continuous improvement efforts, identifying areas for optimization and implementing changes to enhance efficiency, quality, and knowledge sharing.
- They are responsible for managing change processes, ensuring smooth transitions, and minimizing disruptions.
Authority and Decision-Making
- Most of these roles have a certain level of authority and decision-making power within their respective domains.
Collaboration and Knowledge Sharing
- Effective collaboration and knowledge sharing are essential for these roles to succeed.
While these roles have distinct responsibilities and focus areas, they share common goals of ensuring compliance, managing risks, driving continuous improvement, and leveraging subject matter expertise to achieve organizational objectives and maintain high standards of quality and safety. They are more similar than dissimilar and should be looked at holistically within the organization.