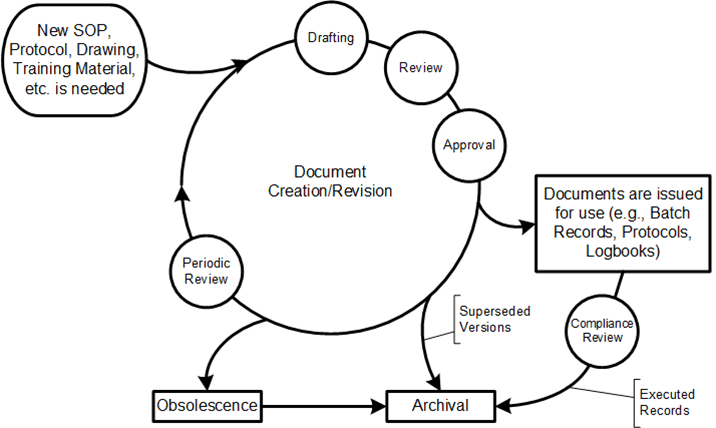
Maintaining high-quality products is paramount, and a critical component of ensuring quality is implementing a robust review of work by a second or third person, a peer review, and/or quality review—also known as a work product review process. Like many tools, it can be underutilized. It also gets to the heart of the question of Quality Unit oversight.
Introduction to Work Product Review
Work product review systematically evaluates the output from various processes or tasks to ensure they meet predefined quality standards. This review is crucial in environments where the quality of the final product directly impacts safety and efficacy, such as in pharmaceutical manufacturing. Work product review aims to identify any deviations or defects early in the process, allowing for timely corrections and minimizing the risk of non-compliance with regulatory requirements.
Criteria for Work Product Review
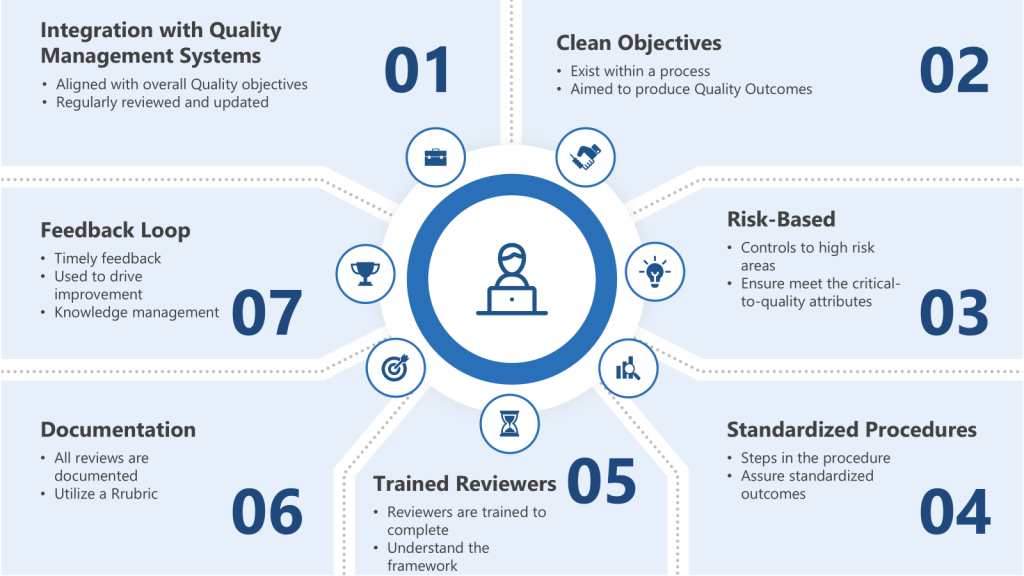
To ensure that work product reviews are effective, several key criteria should be established:
- Integration with Quality Management Systems: Integrate risk-based thinking into the quality management system to ensure that work product reviews are aligned with overall quality objectives. This involves regularly reviewing and updating risk assessments to reflect changes in processes or new information.
- Clear Objectives: The review should have well-defined objectives that align with the process they exist within and regulatory requirements. For instance, in pharmaceutical manufacturing, these objectives might include ensuring that all documentation is accurate and complete and that manufacturing processes adhere to GMP standards.
- Risk-Based: Apply work product reviews to areas identified as high-risk during the risk assessment. This ensures that resources are allocated efficiently, focusing on processes that have the greatest potential impact on quality.
- Standardized Procedures: Standardized procedures should be established for conducting the review. These procedures should outline the steps involved, the reviewers’ roles and responsibilities, and the criteria for accepting or rejecting the work product.
- Trained Reviewers: Reviewers should be adequately trained and competent in the subject matter. This means understanding not just the deliverable being reviewed but the regulatory framework it sits within and how it applies to the specific work products being reviewed in a GMP environment.
- Documentation: All reviews should be thoroughly documented. This documentation should include the review’s results, any findings or issues identified, and actions taken to address these issues.
- Feedback Loop: There should be a mechanism for feedback from the review process to improve future work products. This could involve revising procedures or providing additional training to personnel.
Bridging the Gap Between Work-as-Imagined, Work-as-Prescribed, and Work-as-Done
Work product review is a systematic process that evaluates the output from various tasks to ensure they meet predefined quality standards connecting to work-as-imagined, work-as-prescribed, and work-as-done. Work product review serves as a bridge between these concepts by systematically evaluating the output of work processes. Here’s how it connects:
- Alignment with Work-as-Prescribed: Work product review ensures that outputs comply with established standards and procedures (work-as-prescribed), helping to maintain regulatory compliance and quality standards.
- Insight into Work-as-Done: Through the review process, organizations gain insight into how work is actually being performed (work-as-done). This helps identify any deviations from prescribed procedures and allows for adjustments to improve alignment between work-as-prescribed and work-as-done.
- Closing the Gap with Work-as-Imagined: By documenting and addressing discrepancies between work-as-imagined and work-as-done, work product review facilitates communication and feedback that can refine policies and procedures. This helps to bring work-as-imagined closer to the realities of work-as-done, improving the effectiveness of quality oversight.
Work product review is essential for ensuring that the quality of work outputs aligns with both prescribed standards and the realities of how work is actually performed. By bridging the gaps between work-as-imagined, work-as-prescribed, and work-as-done, organizations can enhance their quality management systems and maintain high standards of quality, safety and efficacy.
Aligning to the Role of Quality Unit Oversight
While work product review does not guarantee Quality Unit Oversight, it is a potential control to ensure this oversight.
In the pharmaceutical industry, the Quality Unit plays a pivotal role in ensuring drug products’ safety, efficacy, and quality. It oversees all quality-related aspects, from raw material selection to final product release. However, the Quality Unit must be enabled appropriately and structured within the organization to effectively exercise its authority and fulfill its responsibilities. This blog post explores what it means for a Quality Unit to have the necessary authority and how insufficient implementation of its responsibilities can impact pharmaceutical manufacturing.
Responsibilities of the Quality Unit
Establishing and Maintaining the Quality System: The Quality Unit must set up and continuously update the quality management system to ensure compliance with GxPs and industry best practices.
Auditing and Compliance: Conduct internal audits to ensure adherence to policies and procedures, and report quality system performance metrics.
Approving and Rejecting Components and Products: The Quality Unit has the authority to approve or reject components, drug products, and packaging materials based on quality standards.
Investigating Nonconformities: Ensuring thorough investigations into production errors, discrepancies, and complaints related to product quality.
Keeping Management Informed: Reporting on product, process, and system risks, as well as outcomes of regulatory inspections.
What It Means for a Quality Unit to Be Enabled
For a Quality Unit to be effectively enabled, it must have:
- Independence: The Quality Unit should operate independently of production units to avoid conflicts of interest and ensure unbiased decision-making.
- Authority: It must have the authority to approve or reject the work product without undue influence from other departments.
- Resources: Adequate personnel are essential for conducting the quality unit functions.
- Documentation and Procedures: Clear, documented procedures outlining responsibilities and processes are crucial for maintaining consistency and compliance.
Insufficient Implementation of Responsibilities
When a Quality Unit insufficiently implements its responsibilities, it can lead to significant issues, including:
- Regulatory Noncompliance: Failure to adhere to GxPs and regulatory standards can result in regulatory action.
- Product Quality Issues: Inadequate oversight can lead to the release of substandard products, posing risks to patient safety and public health.
- Lack of Continuous Improvement: Without effective quality systems in place, opportunities for process improvements and innovation may be missed.
The Quality Unit is the backbone of pharmaceutical manufacturing, ensuring that products meet the highest standards of quality and safety. By understanding the Quality Unit’s responsibilities and ensuring it has the necessary authority and resources, pharmaceutical companies can maintain compliance, protect public health, and foster a culture of continuous improvement. Inadequate implementation of these responsibilities can have severe consequences, emphasizing the importance of a well-structured and empowered Quality Unit.
By understanding these responsibilities, we can take a risk-based approach to applying quality review.
When to Apply Quality Review as Work Product Review
Work product review by Quality should be applied at critical stages to guarantee critical-to-quality attributes, including adherence to the regulations. This should be a risk-based approach. As such, it should be identified as controls in a living risks assessment and adjusted (add more, remove where unnecessary) as appropriate.
Closely scrutinize the responsibilities of the Quality Unit in the regulations to ensure all are met.
Best Practices in Quality Review
Rubrics are a great way to standardize quality reviews. If it is important enough to require a work review, it is important enough to standardize. The process owner should develop and maintain these rubrics with an appropriate group of stakeholder custodians. This is a key part of knowledge management. Having this cross-functional perspective on the output and what quality looks like is critical. This rubric should include:
- Definition of prescribed work and the intended output that is being reviewed
- Potential outcomes related to critical attributes, including definitions of technical accuracy
- Methods and techniques used to generate the outcome
- Operating experience and lessons learned
- Risks, hazards, and user-centered design considerations
- Requirements, standards, and code compliance
- Planning, oversight, and acceptance testing
- Input data and sources
- Assumptions
- Documentation required
- Reviews and approvals required
- Program or procedural obstacles to desired performance
- Surprise situations, for example, unanticipated risk factors, schedule or scope changes, and organizational issues
- Engineering human performance tool(s) applicable to activities being reviewed.
The rubric should have an assessment component, and that assessment should feed back into the originator’s qualified state.
Work product reviews must be early enough to allow feedback into the normal work for repetitive tasks. This should lead to gates in processes, quality-on-the-floor, or better-trained supervisors performing better and more effective reviews. This feedback should always be to the responsible person – the originator—and should be, wherever possible, face-to-face feedback to resolve the particular issues identified. This dialogue is critical.
Conclusion
Work product review is a powerful tool for enhancing quality oversight. By aligning this process with the responsibilities of the Quality Unit and implementing best practices such as standardized rubrics and a risk-based approach, companies can ensure that their products meet the highest standards of quality and safety. Effective work product review not only supports regulatory compliance but also fosters a culture of continuous improvement, which is essential for maintaining excellence in the pharmaceutical industry.